| Feb 04, 2014 |
Scientists turn primitive artificial cell into complex biological materials
|
|
(Nanowerk News) It is a big dream in science: To start from scratch with simple artificial microscopic building blocks and end up with something much more complex: living systems, novel computers or every-day materials. For decades scientists have pursued the dream of creating artificial building blocks that can self-assemble in large numbers and reassemble to take on new tasks or to remedy defects. Now researchers from University of Southern Denmark have taken a step forward to make this dream come true.
|
|
“The potential of such new man-made systems is almost limitless, and many expect these novel materials to become the foundation of future technologies”, says Dr. Maik Hadorn from Department of Chemistry and Applied Biosciences at ETH Zürich, who conducted the research as a postdoctoral research fellow at University of Southern Denmark (SDU).
|
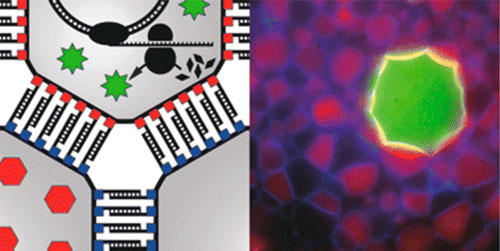 |
| The spatially controlled DNA-directed bottom-up synthesis of complex microassemblies and macroassemblies of giant unilamellar vesicles functionalized with a basic cellular machinery to express green fluorescent protein and specified neighbor-to-neighbor interactions. The researchers show both that the local and programmable DNA pairing rules on the nanoscale are able to direct the microscale vesicles into macroscale soft matter assemblies and that the highly sensitive gene-expression machinery remains intact and active during multiple experimental steps. An in silico model recapitulates the experiments performed in vitro and covers additional experimental setups highlighting the parameters that control the DNA-directed bottom-up synthesis of higher-order self-assembled structures. (© American Chemical Society)
|
|
Over the last three years he and the colleagues Eva Boenzli, Kristian T. Sørensen and Martin M. Hanczyc from the Center for Fundamental Living Technology (FLinT) at SDU have worked on the challenges of making primitive building blocks assemble and turn into something functional.
|
|
“We used short DNA strands as smart glue to link preliminary stages of artificial cells (called artificial vesicles) to engineer novel tissue-like structures”, says Dr. Maik Hadorn.
|
|
As part of the EU-sponsored project MATCHIT (MATrix for CHemical Information Technology) Dr. Maik Hadorn and coworkers have earlier showed that short DNA strands can guide the self-assembly process of artificial vesicles; that two types of artificial vesicles can be linked in a way predefined by the person conducting the experiment, and that assembled structures can be reassembled, when triggered externally ("Specific and reversible DNA-directed self-assembly of oil-in-water emulsion droplets").
|
|
In their most recent scientific article ("Defined DNA-Mediated Assemblies of Gene-Expressing Giant Unilamellar Vesicles"), published in Langmuir in December 2013, the researchers from SDU, in collaboration with colleagues from Italy and Japan, not only increased the complexity of the self-assembled structures that are now composed of several types of artificial vesicles – they also loaded one vesicle type with a basic cellular machinery derived from bacterial cells. This enabled these vesicles to translate an encapsulated genetic blueprint into a functional protein.
|
|
Put together the researchers have managed to engineer controlled assemblies that are visible to the naked eye and that resemble natural tissues in their architecture as well as in their functionalities.
|
|
Methods of constructing simple artificial structures have been known for decades, but only the use of DNA strands that act as a smart glue has allowed the researchers to overcome shortcomings of precedent methods and to engineer higher-order structures of predefined and programmable architecture.
|
|
“As the artificial vesicles resemble natural cells both in size and composition, they are an ideal starting point for a multitude of applications. One application can be a temporal support for wound healing: A wound may be covered with assemblies of vesicles that are tailored in a patient specific manner. They will not only protect the natural cells beneath the wound but also initiate and guide the differentiation of these cells so that they divide and differentiate. Finally, these regenerated natural cells can take over and fulfill their protective function”, explains Maik Hadorn.
|
|
The new systems are also of value in studying cells: “Natural organisms are complex. Simple model systems like our tissue-like structures may help to reveal secrets for example of cell communication and cell differentiation”.
|
|
Besides these two potential applications in personalized medicine and natural sciences, one can also think of using assembled vesicles as small bioreactors.
|
|
“It’s somehow like cooking”, Dr. Hadorn explains: “If you’re preparing a meal, most of the time you’re not using just one pot. To prepare your meat, potatoes, and also the vegetables in just one pot is almost impossible. By using different pots you’re making sure that the conditions for the preparation of each component are optimal and that the components only meet if they are ready. Transferred to current one-pot bioreactors in science we often face similar problems. However, by using microscopic pots (i.e. vesicles) that are loaded with a defined set of substances and that are in close proximity to one another, one can think of microscopic bioreactors in which gates open to release substances from one vesicle into a neighboring vesicles. This ensures that the reaction conditions are optimal for the synthesis of products too complex for today’s one-pot bioreactors.”
|

