| Dec 14, 2023 |
Fully recyclable 3D printing breakthrough uses microwaves to enable sustainable additive manufacturing
|
|
(Nanowerk News) Additive manufacturing, commonly known as 3D printing, has shown immense promise as an engine for modern manufacturing transformation. By rapidly printing complex objects on demand, the technology has disrupted industries from aerospace to medical devices. However, an urgent roadblock has threatened the sustainability of wider adoption across consumer and industrial sectors alike: plastic waste.
|
|
Most commercially available materials for 3D printing today, from cheap PLA plastics to high-end polyamides, share two troubling traits after printing – they are not recyclable and pose dangers from pollution. That's because these plastics contain highly stable carbon-carbon polymer backbones resistant to cost-effective recycling methods once cured into products. As global volumes of 3D printed plastic parts have skyrocketed 1500% over the last decade, vast piles of post-industrial and post-consumer waste with no recycling pathway have built up behind the technology’s celebrated rise.
|
|
For years experts have pointed to an alluring alternative – reversible polymers that can rearrange their covalent bonds when exposed to certain stimuli, enabling repeat processing, reprinting and full recycling. Also called dynamic covalent polymer networks or covalent adaptable networks (CANs), these transformable materials have remained just out of viable reach for reliable 3D printing despite intense R&D attention.
|
|
Until now. Groundbreaking new research from scientists in Israel introduces reversible polymers tailored for 3D printing that can be fully recycled using only a standard microwave oven. This pioneering methodology finally unlocks the promise of sustainably printing objects on demand at scale without toxic waste piling up our landfills and oceans.
|
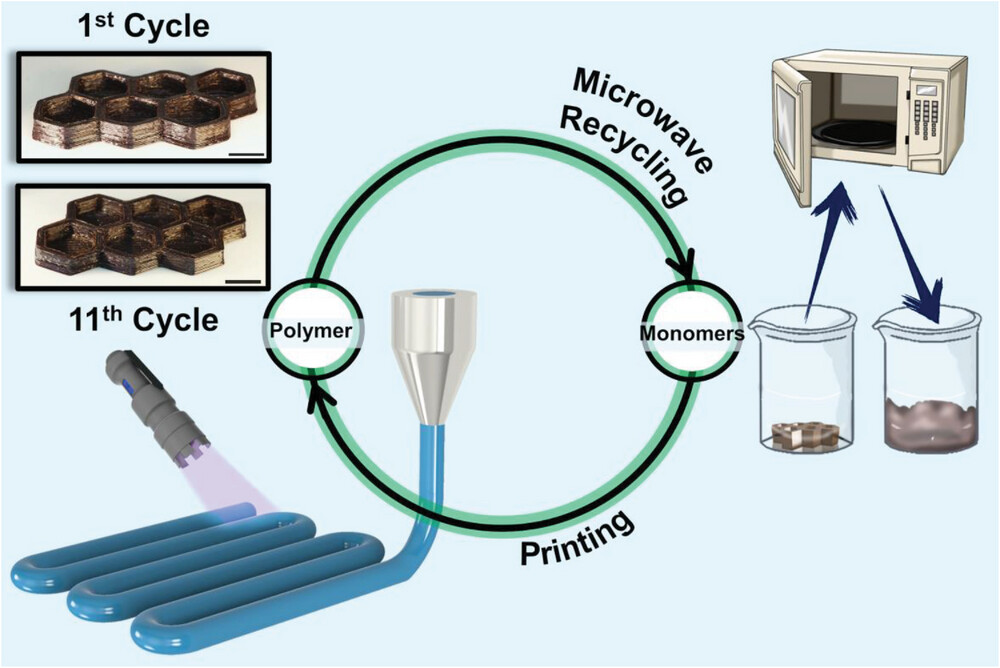 |
| A schematic representation of this work's idea: printing the monomers into polymers using UV light, then recycling them into monomers using a microwave oven, and then reprinting them. Both the 1st printing cycle and 11th were printed under the same conditions (70 °C, 365 nm continuous irradiation), showing no significant differences in the resolution. All scale bars are of 1 cm. (Reprinted with permission by Wiley-VCH Verlag)
|
|
The findings have been published in Advanced Materials ("Fully Recyclable Cured Polymers for Sustainable 3D Printing").
|
|
Led by Professor Shlomo Magdassi, the researchers developed fully recyclable and reprintable polymeric compositions for fused filament fabrication 3D printing at much lower temperatures. This is achieved through purposeful design of polymers undergoing reversible photopolymerization via cycloaddition reactions that form new bonds when exposed to UV light.
|
|
Critically, an everyday microwave oven can then rapidly depolymerize the polymers for complete recycling. This enables multiple printing cycles without compromising mechanical properties or needing to add any new materials.
|
|
At the core of this new approach lies the design of a custom monomer containing triethylenetetramine and cinnamaldehyde groups. This monomer can form cycloadducts through [2+2] and [4+4] cycloaddition reactions under 365nm UV irradiation. The presence of a specialized tin-based catalyst developed by the researchers accelerates these reversible photopolymerization reactions.
|
|
Printing is conducted via direct ink writing 3D printing methodology, with the UV light causing simultaneous polymerization and crosslinking to retain the desired printed shape. Remarkably, the printing temperature is only 70 °C – 50 °C lower than the previously reported lowest temperature RCBP printing method.
|
|
According to Dr. Hanna Dodiuk, “this breakthrough will facilitate using sustainable raw materials, ultimately contributing to a more efficient and eco-friendly perspectives for 3D printing.”
|
|
To recycle the printed parts, the researchers make an innovative use of microwave radiation. Just 10 minutes in a standard microwave oven at a power of 216W caused liquification and 97.6% reversion back to the original functional monomers. While microwave exposure did heat samples to 180 °C, heating alone at this temperature did not enable recycling. This suggests unique microwave effects are enabling accelerated bond reversion.
|
|
As Dr. Dodiuk elaborates, “Typically, reversing cycloaddition reactions requires irradiation at the harmful range of UVC (<260 nm for cinnamaldehyde-based moieties). The instability of four and eight-membered rings resulting from bond angle stress makes them susceptible to ring-opening via excitation under UVC.”
|
|
“An alternative approach was explored to avoid UVC irradiation, based on microwave irradiation (25–38 mm), which is known to cause rapid heating through the rotation of polar molecules. Some cycloaddition reactions can dissociate under high temperatures that cause vibrations of the rings, leading to steric stresses, which results in conversion to the more sterically stable form – the original molecules”.
|
|
Remarkably, the researchers successfully demonstrated 11 integrated print-recycle cycles with no loss in mechanical or thermal properties. Tensile strength, elongation at break, and glass transition temperature remained effectively unchanged between the first and eleventh iterations.
|
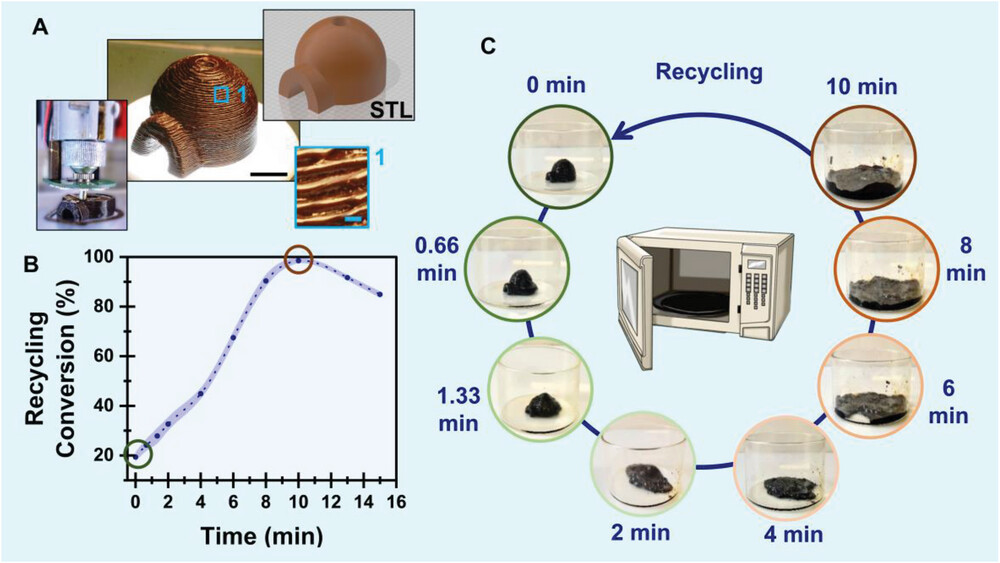 |
| Recycling the polymer in a microwave oven. A) A printed sample with overhanging parts. The black scale bar represents 1 cm, whereas the pale blue represents 0.5 mm. The figure shows the STL file, the printed layer-by-layer structure, and magnification of a section of the object emphasizing the layer structure and dimensions. B) Conversion of the adducts’ dissociation (recycling conversion, percentage of the recovered double bonds) as a function of microwave irradiation time. The pale blue shade represents the error bars. The conversion was calculated based on the changes in the samples’ absorbance and fluorescence. C) pictures of postcured samples after different irradiation time intervals in a microwave oven (216 W). (Reprinted with permission by Wiley-VCH Verlag)
|
|
Complete dissolution of printed parts after microwave recycling confirmed excellent reversibility. The researchers also analyzed polymer conversion at each stage using NMR studies and UV-vis spectrophotometry to quantify recycling efficiency.
|
|
The approach enables sustainable additive manufacturing with widespread potential applications, from electronics to biomedical devices. It overcomes key barriers that have challenged previous efforts to apply reversible polymers to 3D printing over the past decade.
|
|
As Professor Shlomo Magdassi summarizes, “this research presents a sustainable approach to 3D radiation printing by utilizing reversible cycloaddition reactions. The new synthesized monomer demonstrates printability at significantly lower temperatures than previously reported RCBPs. The presented approach allows multiple printing cycles without compromising the printed objects’ mechanical and thermal properties and without replenishing materials.”
|
|
With further development, this pioneering methodology could finally unlock the promise of fully recyclable 3D printable plastics. Given the vast volumes of plastic waste challenging communities across the world, more sustainable materials science breakthroughs like this have never been more crucial. Professor Magdassi believes this research represents “a promising step towards advancing sustainability, polymers, and material science.”
|


