| Posted: Apr 09, 2009 | |
Synthetic DNA nanomachines go to work inside living cells |
|
| (Nanowerk Spotlight) DNA nanotechnology has gained lots of attention recently, and sensationally titled articles like "Nanotechnology may have found its Henry Ford – tiny DNA robots could be the future of assembly lines" are certainly pushing the topic into the public awareness. As a matter of fact, scientists like Ned Seeman, who is the subject of the article, have worked for years in this area and a large number of DNA-based nanomechanical devices have been described in the literature. Also see our Spotlight "The gripping potential of DNA nanotechnology". | |
| DNA nanomachines – synthetic DNA assemblies that switch between defined molecular shapes upon stimulation by external triggers – can be controlled by a variety of methods; these include pH changes and the addition of other molecular components, such as small molecule effectors, proteins and DNA strands. | |
| A team of Indian researchers has now taken structural DNA nanotechnology, where so far only in vitro applications have been demonstrated, across a new boundary and into living systems. In an article in this week's online edition of Nature Nanotechnology, they describe the successful operation of an artificially designed DNA nanomachine inside living cells, and show that these nanomachines work as efficiently inside cells as in vitro (A DNA nanomachine that maps spatial and temporal pH changes inside living cells). | |
| "We named our DNA nanomachine the I-switch" Yamuna Krishnan tells Nanowerk. "The device is externally triggered by protons and functions as a pH sensor based on fluorescence resonance energy transfer (FRET) inside living cells. We demonstrate the ability of this DNA nanomachine to function inside living cells by using it to map spatio-temporal pH changes associated with endosome maturation. Our studies show that it is able to function as efficiently inside the endosome of a living cell as it does in a test tube." | |
| Krishnan is a scientist at the National Centre for Biological Sciences in Bangalore, India, where she heads the Chemical Biology Group. The DNA assembly developed by her team is a robust pH-triggered nanoswitch with reasonably fast response times, sustained efficiency over several cycles, and a working cycle that does not generate toxic byproducts (the byproducts of a complete cycle for the I-switch are salt and water). | |
 |
|
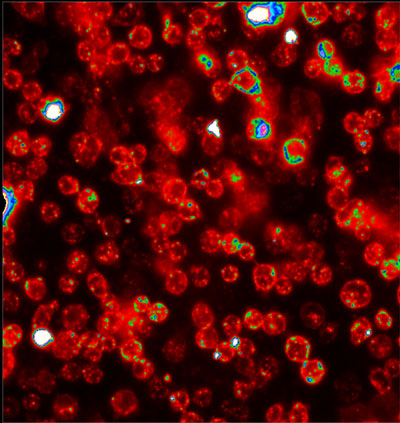
| Drosophila cells with the I-switch inside their endosomes. (Image: Dr. Krishnan, National Centre for Biological Sciences) | |
| The I-switch has a pH sensitivity between pH 5.5 and 6.8 – which is ideal for monitoring changes in intracellular pH – and offers complementary information to that obtained through the use of small-molecule fluorescent pH probes. Krishnan points out that, unlike such pH probes, the I-switch is a FRET-based sensor that is equally bright at both physiological and acidic values of pH. | |
| "Most importantly, with pH probes based on green fluorescent proteins or small molecules one is limited by a fixed wavelength, but the I-switch, which is an artificially designed DNA scaffold, can incorporate any appropriate FRET pair" she says. "It can therefore be used to simultaneously follow multiple proteins, with each protein bearing a distinct FRET pair, thus defining it as a powerful probe to study compartment mixing in intracellular sorting or trafficking events." | |
| As of now, the switch only gives a temporal resolution of 5 minutes with response times of 1-2 minutes. However this can be solved with new and improved designs, something the Indian team is already working on. Faster DNA switches will be able to measure pH changes on shorter timescales, as well as over different ranges of pH sensitivity, | |
| According to Krishnan, it is notable that, in analogy to cellular machines, artificially designed DNA nanomachines are responsive to a molecular cue, can be specifically coupled to the cellular environment, and yet function independently within the crowded cellular milieu. "Thus, DNA nanomachine function on the nanoscale can be efficiently transduced to cellular length scales" she says. | |
| "Clearly, there is a lot of potential to use nucleic acid scaffolds as sensors for various biomolecular cues within living systems" says Krishnan. "The next frontier for structural DNA nanotechnology inside living systems will be sensing newer and newer molecules, and future frontiers will go from just passively reporting on cellular signaling, as we have shown, to manipulating and tuning cell signaling. " | |
| As for their current work, Krishnan says: "We just want this sensor to be used and to showcase the potential of DNA scaffolds as high-performance sensors. We are happy to ship out the sensor to anyone who wants to use it, without needing to collaborate at all." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
