| Posted: Dec 06, 2006 | |
Quite unexpectedly, gold, silver and copper can produce single-walled carbon nanotubes |
|
| (Nanowerk Spotlight) The controlled synthesis of single-walled carbon nanotubes (SWCNTs), which generally requires a nanoscale catalyst metal, is crucial for their application to nanotechnology. In the chemical vapor deposition (CVD) of SWCNTs, the known effective catalyst species are the iron-family elements iron, cobalt, and nickel, with which a high SWCNT yield can be obtained. However, gold, silver, and copper have never been reported to produce SWCNTs. It is well known that iron, cobalt, and nickel have the catalytic function of graphite formation but that gold does not. The difference between the iron-family metals and gold is that the binding energy of carbon is much larger for the iron-family metals. Carbon atoms cannot stay on gold long enough to form a graphitic network. Thus, it is rather natural for iron, cobalt, and nickel to generate SWCNTs, but it is totally unexpected that gold would produce them too. The same picture is applicable to silver and copper. Nevertheless, researchers in Japan succeeded in developing a nanoparticle activation method that shows that even gold, silver, and copper act as efficient catalysts for SWCNT synthesis. These non-magnetic catalysts could provide new routes for controlling the growth of SWCNTs. | |
| "We have investigated the role of catalysts in CVD of SWCNT, and aimed at uncovering the SWCNT growth mechanism" Yoshikazu Homma explains to Nanowerk. "The known effective catalyst species of SWCNT synthesis in CVD are almost limited to the iron-family transition metals iron, cobalt and nickel, and it came as a total surprise for us that gold and silver would produce SWCNTs, too." | |
| One particular focus of Professor Homma's laboratory at the Tokyo University of Science is carbon nanotube growth. With this new research they have now demonstrated that any metal, even gold and silver, when properly treated, can act as the catalyst for SWCNT synthesis in chemical vapor deposition. And the treatment is actually simple: just pre- heating the metal nanoparticles in air to 800°C or higher, and after that CVD growth starts immediately. | |
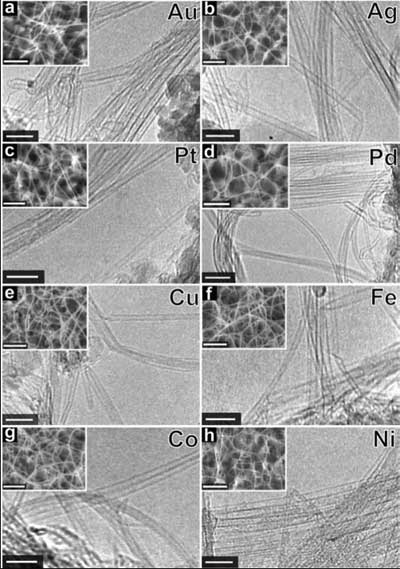 |
TEM and SEM (insets) micrographs of SWCNTs grown on Al-hydroxide film using various metal species as catalyst. (a) Au. (b) Ag. (c) Pt. (d) Pd. (e) Cu. (f) Fe. (g) Co. (h) Ni. The scale bars for TEM and SEM images are 10 and 200 nm, respectively. (Reprinted with permission from American Chemical Society) |
| A paper on these findings, titled "Single-Walled Carbon Nanotube Growth from Highly Activated Metal Nanoparticles", was published in the November 23, 2006 online edition of Nano Letters. | |
| "We asked ourselves 'why could these elements produce SWCNTs?'" says Homma. "Copper and noble metals with nanoscale diameter might have a function similar to that of the iron family metals, but it is more likely that SWCNTs might be formed from nanosized particles without such a function. A possible explanation is that these metal particles are not in a crystal state. Instead, they have a cluster-like structure, and carbon atoms are soluble in them. Then, carbon atoms might precipitate to cover the surface of the nanoparticle, resulting in the formation of a hemispherical cap with a graphitic structure as the precursor of SWCNT growth. We think that the essential role of metal particles is to provide a platform on which carbon atoms can form a hemispherical cap. Then, SWCNTs grow in a self-assembled fashion." | |
| This is important because the conventional iron-family metals are ferromagnetic, which fact makes it difficult to evaluate the magnetic properties of SWCNTs or to apply them to superconducting research. | |
| "Our findings provide non-magnetic catalysts" Homma points out. "We also hope that SWCNTs can be grown on a metal substrate by using the new catalyst species. So far, it was difficult to directly synthesize SWCNTs on metals because of alloying of the iron-family metals with the substrate metal." | |
| Controlled synthesis of SWCNT's length, diameter and especially chilarity still poses a significant challenge for researchers and materials engineers. The electrical and optical properties of the SWCNT depend on its chilarity. Thus, chilarity control is the most important issue for the electronic/photonic applications of SWCNTs. By diversifying the catalyst species the researchers hope to find a catalyst suitable for better control of SWCNT synthesis. | |
| "Nanocatalysts are the key to determining chirality" says Homma. The new findings made in his lab will contribute to diversifying SWCNT catalysts as well as to the investigation of SWCNT growth mechanism. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
