| Posted: Oct 27, 2009 | |
Self-powered smart window |
|
| (Nanowerk Spotlight) From an energy savings point of view, the use of smart windows – electrically switchable glass which controls the amount of light passing through when voltage is applied – can save costs for heating, air-conditioning and lighting and avoid the cost of installing and maintaining motorized light screens or blinds or curtains. A disadvantage is of course the fact that the smart windows themselves need to draw energy in order to do their job. Now, researchers have developed a self-powered, fast-switching smart window that doubles as a solar cell, using sun light to power its chromic behavior and making the case for energy savings even more compelling. | |
| Today, the most common materials for smart windows are electrochromic (color change upon exposure to electric charge) and photochromic (color change upon exposure to light) materials with reversible transmittance in response to an applied voltage or illumination. | |
| Back in 1996, researchers already demonstrated a self-controlled and self-powered photoelectrochromic system – the photoelectrochromic cell (PECC) – that integrates the photovoltaic and electrochromic elements into a single device. A PECC darkens when exposed to sunlight and clears when no longer sunlit but the process is rather slow. PECCs have a configuration similar to that of dye-sensitized solar cells (DSSCs) since both devices employ a dye-sensitized titanium nanoparticle nanoparticle film as the photoanode, but the platinum counter- electrode of DSSCs is replaced by tungsten oxide (WO3) electrochromic film in PECC. | |
| By employing a patterned tungsten oxide/platinum electrochromic electrode and a dye-sensitized titanium dioxide nanoparticle photoanode, researchers in Taiwan have now demonstrated a self-powered photovoltachromic cell (PVCC) which exhibits distinct electrochromic characteristics of a fast switching rate and tunable transmittance under illumination. The novel device has both photoelectrochromic and photovoltaic characteristics. | |
| "Under light illumination, the PVCC can be colored at short circuit with tunable transmittance and it can be bleached exceedingly fast by simply opening the circuit," says Jih-Jen Wu, a researcher at the Department of Chemical Engineering at National Cheng Kung University in Tainan. "This unique performance results from the crucial charge transfer pathways provided by the patterned tungsten oxide/platinum electrochromic electrode." | |
| Wu is first author of a recent paper in ACS Nano ("Fast-Switching Photovoltachromic Cells with Tunable Transmittance") in which the research team proposes PVCC as a scheme for constructing a self-powering, fast-response, transmittance-tunable smart window, and for constructing a self-powering, contrast-adjustable display when the PVCC cells are fabricated pixel-by-pixel. | |
| For their experiments, the researchers fabricated two devices: Their proposed PVCC with a patterned tungsten oxide/platinum film as the electrochromic electrode and a PECC with the same configuration as that demonstrated in 1996, both using identical tungsten oxide, photoelectrodes and electrolytes. | |
 |
|
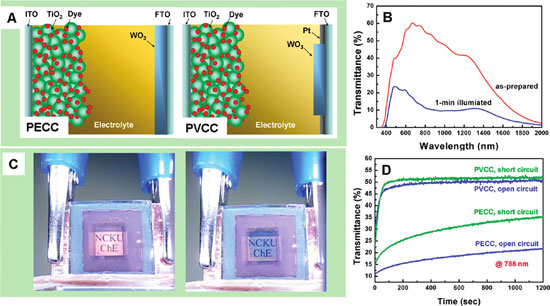
| (A) Schematics of PECC and PVCC. (B) Transmittance spectra of PECC in the as-prepared and the colored states, measured at open circuit. (C) Photographs of PVCC in the as-prepared (left) and colored (right) states. The colored photograph was taken at short circuit under illumination. The illumination makes the background letters rather clear even though the PVCC is colored. (D) Evolutions of cell bleaching at a wavelength of 788 nm for open- and short-circuited PECC and PVCC in the dark. (Reprinted with permission from American Chemical Society) | |
| The results show that PVCC indeed has a significantly faster response of cell bleaching and that the transmittance of PVCC in the colored state is higher than that of PECC. In addition, Figure D above also illustrates that, compared to bleaching at open circuit, the bleaching rate is substantially faster at short circuit for PECC but the difference is not as significant for PVCC. | |
| According to Wu, the patterned tungsten oxide/platinum electrode brings platinum into contact with tungsten oxide and allows both platinum and tungsten oxide to transfer charges to the electrolyte individually. | |
| "The charge transfer between platinum and the electrolyte gives PVCC significant photovoltaic characteristics" he says. "The fast open-circuit bleaching under illumination is achieved by the positive potential on the tungsten oxide/platinum electrochromic electrode and the tungsten oxide/platinum-electrolyte channel, which facilitates the discharge of lithium ions and electrons, respectively, from the colored tungsten oxide to the electrolyte. The transmittance of PVCC can be tuned by modulating the number of lithium ions and electrons into tungsten oxide, which is achieved by controlling the operating current and voltage on the tungsten oxide/platinum terminal by driving an external load of variable resistance." | |
| This method can also be applied to build a self-powering and contrast-adjustable display when the PVCC cells are fabricated pixel-by-pixel. As a result, the proposed fast-response, energy-efficient, and transmittance-tunable PVCC has great potential for smart window and display applications. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
