| Posted: Dec 04, 2009 | |
Improved nanotechnology catalysts bring clean energy applications closer |
|
| (Nanowerk Spotlight) It has been almost 200 years since chemists stumbled across the fact that certain chemicals can speed up a chemical reaction – a process, now known as catalysis, that has become the foundation of the modern chemical industry. By some estimates 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture. Catalysis is the acceleration of a chemical reaction by means of a substance, called a catalyst, which is itself not consumed by the overall reaction. | |
| The most effective catalysts are usually transition metals or transition metal complexes. The increasing role that nanotechnology is playing in modern catalysis has to do with two main effects of catalysts that have only nanoscale dimensions. On one hand, the increasing surface-to-volume ratio with decreasing particle size strongly increases the specific catalytic activity, while on the other hand quantum confinement effects can completely alter the chemical characteristics of sufficiently small nanoparticles. Researchers have also shown that the atomic characteristics of metallic nanoparticles, including particle size and surface composition, are critical to catalytic activity and selectivity. | |
| Nanotechnology catalytical techniques are having a profound impact on clean energy research and development, ranging from hydrogen and liquid fuel production to clean combustion technologies. In this area, catalyst stability is paramount for technical application, and remains a major challenge, even for many conventional catalysts. | |
| Thermal stability in particular is a challenge across many currently discussed technical applications and an obstacle for many nanocatalyst-enabled devices, from sensors to fuel production. In particular fuel processing technologies (hydrogen and/or liquid fuel production from fossil and renewable resources, clean combustion) typically proceed at particularly severe conditions (high temperatures, high through-put, contaminated fuel streams, etc) and hence require particular attention to catalyst stabilization, but even many processes at much lower temperature conditions, such as fuel cells, are still looking for catalysts with improved stability. | |
| In essence, the poor thermal stability of many nanomaterials currently constitutes probably one of the main challenges on the way to technical application of many nanomaterials. | |
| Researchers have now overcame a major hurdle in developing more efficient nanoparticle catalysts by demonstrating high-temperature stability in metallic nanoparticles. | |
| "We have worked for a while on stabilizing metal nanoparticles via embedding or encapsulation in oxide matrices," Götz Veser, an associate professor and CNG Faculty Fellow of chemical and petroleum engineering in Pitt's Swanson School of Engineering, tells Nanowerk. "However, these methods are difficult – and sometimes impossible – to apply to very small nanoparticles in the size range below 5 nm. However, many of the exciting novel nanosize effects occur exactly in this regime, and we hence needed to find a different, hopefully easier and more efficient way to stabilize metal nanoparticles in this regime." | |
| Veser and his group created metal-alloy particles in the range of 4 nanometers that can withstand temperatures of more than 850 degrees Celsius, at least 250 degrees more than typical metallic nanoparticles. The team has reported their findings in the November 29, 2009 online issue of Nature Materials ("Exceptional high-temperature stability through distillation-like self-stabilization in bimetallic nanoparticles"). | |
 |
|
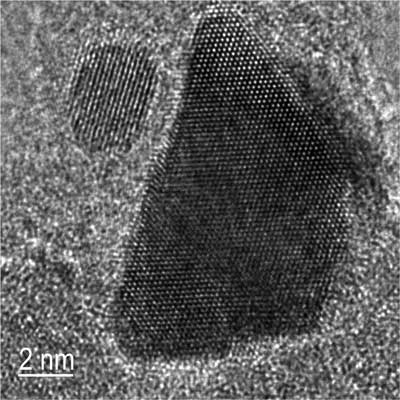
| This TEM image shows the main effect of the “platinum bleeding” in that a small (~4nm) platinum-rhodium alloy particle sits right next to an agglomerated, large (~20nm) pure platinum particle. (Reprinted with permission from Nature Publishing Group) | |
| Forged from the catalytic metals platinum and rhodium, the highly reactive particles work by dumping their heat-susceptible components as temperatures rise, a quality Anmin Cao, the paper's first author, likened to a gecko shedding its tail in self-defense. | |
| Veser explains how this new research offers two novel aspects with regard to synthesizing stable nanomaterials: | |
| "On one hand, it demonstrates an alternate way of stabilizing metal nanoparticles. Efforts to-date, including in our own group, have typically focused on thermal stabilization of metal nanoparticles by embedding them into a thermally stable oxide matrix – either by direct encapsulation, or by embedding in the pore network of microporous oxides. In our present work, we approach the problem from the angle of thermal stabilization by modification of the metal nanoparticle itself. This makes the results generally independent of the support, and hence broadly applicable." | |
| "On the other hand, our results also improve our understanding of the stability of metal particles in the nanosize regime. While it was fairly predictable that one should observe a thermal stabilization when doping a metal with a second, higher melting-point metal, the 'distillation-like' self-stabilization that we observed is surprising. It opens up a new understanding of the dynamics of the melting/de-alloying process on the nanoparticle level. At the same time, again from an applied perspective, it also results in a more flexible stabilization mechanism, since it makes the particles robust against temperature fluctuations in a process." | |
| The work behind this Nature Materials paper opens some exciting new research avenues, which Veser's team are currently investigating. These relate to questions such as "how to glue the gecko's tail back on", i.e. is there a way to revert the de-alloying process, and how is this de-alloying process affected by the shape of the nanoparticle, nanoconfinement, distribution of the two metals within the nanoparticle, etc. | |
| "In general, we feel that we have just pushed open the door to a whole range of exciting new questions about the nature of materials behavior on the nanolevel" says Veser. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
