| Posted: Dec 21, 2009 | |
Unique nanomechanical response of DNA allows high-speed direct digital detection |
|
| (Nanowerk Spotlight) Much of today's genetic research and diagnostics uses tools and technologies enabled by DNA's ability to bind to its complementary strand in a sequence specific manner. By placing short stands of DNA with known sequences on a surface and watching if molecules from a biological sample (tissue, blood, etc) bind to these immobilized DNAs allows researchers to determine genetic composition of the sample. This information can help identify mutations responsible for diseases or help guide the doctor to choose the best treatment strategy. For biologists studying molecular mechanisms inside cells, this information helps to quantify the expression levels of genes. Detection of the binding – or hybridization – of DNA strands is at the heart of modern medicine. | |
| The technology for detecting DNA hybridization mainly relies on the use of fluorescent labels. The complementary strand coming from the sample bears a label, so detection of florescence signal indicates hybridization. While this may sound straightforward, it has major limitations. Sometimes, a stand of DNA that does not have a complementary sequence can get stuck on the surface. This would lead to a false positive signal. Sometimes, fluorescent labels bleach and do not provide any signal. This would lead to a false negative signal. In some cases, the biochemical reaction used to attach label molecules does not work properly, so fewer DNA strands might bear labels. In addition, most samples contain some background fluorescence that has nothing to do with DNA. From the instrumentation point of view, it is difficult to detect small and large fluorescence intensities in the same experiment due to technical limitations of optical instruments (biologists are very much interested in detecting both small and large amounts of DNA). | |
| When you add all these things up, it becomes clear that there is a lot of room for advancements in genetic analysis and improvements in this area can have important implications for biological studies and medical applications. | |
| Researchers at Harvard and Stanford University have now reported a new technique for genetic analysis using nanomechanical response of hybridized DNA/RNA molecules. This new technique is several orders of magnitude more sensitive than other approaches published in the literature and it is a lot simpler to use. The Harvard team has demonstrated its utility in clinical samples and discriminated different cancer tissues through their genetic composition. This has important implications for cancer diagnostics and therapy. | |
| Ozgur Sahin, a researcher at the Rowland Institute at Harvard, who led the study, tells Nanowerk that there are two points in his group's recent work that might be of general interest to the nano community: | |
| "1) Detection of DNA for genetic analysis generally uses fluorescent labels. We have discovered that DNA had indeed possessed an intrinsic nanomechanical label. This new label is far more effective than fluorescent labels or other approaches. | |
| "2) Genetic analysis requires cumbersome biochemical processes. Our method eliminates all these process and yet provide much better performance than existing approaches. This means that it can bring the value of genetic tests to the patients right at the doctor's office. At the moment medical samples have to be shipped to laboratories where specialized technicians are employed." | |
| Sahin and his team have reported their findings in the December 13, 2009 online edition of Nature ("DNA nanomechanics allows direct digital detection of complementary DNA and microRNA targets"). | |
| Sahin explains that he and his team had a simple intuition: "If one can measure mechanical properties of molecules in a fast and robust way, it could provide a dramatic improvement in sensitivity and simplification in the detection of hybridization. The reason is that mechanical properties change dramatically upon hybridization. They are unique to the molecule and they change only when new chemical bonds are formed. This can help eliminate all those false positives and negatives that come with fluorescent labels." | |
| "A back of the envelope analysis made in 2002, when we originally thought about this experiment, showed that the limit of detection could be improved by nearly 8 orders of magnitude compared to the state of the art techniques of that time," says Sahin. "This was exciting, but as we interacted with experts in genetic analysis later, we learned that the potential of our approach in simplifying the measurement process was equally important. The path to fluorescent detection incorporates many steps of biochemical reactions involving enzymes that don't give their 100% at all times. It is also laborious and it consumes reagents leading to a financial burden." | |
| Motivated by the potential impact in genetic analysis Sahin worked on a high speed mechanical sensor for his PhD thesis. This instrument was the focus of our Nanowerk Spotlight article back in 2007 ("Feeling your way through the nanoworld"). Once it was operational Sahin and his coauthors focused on the DNA hybridization detection problem. | |
| "In the end we have developed a technique that looks at DNA molecules one by one, mechanically feels their stiffness, and decides whether they are hybridized or not. In a way, it provides digital precision," says Sahin. | |
 |
|
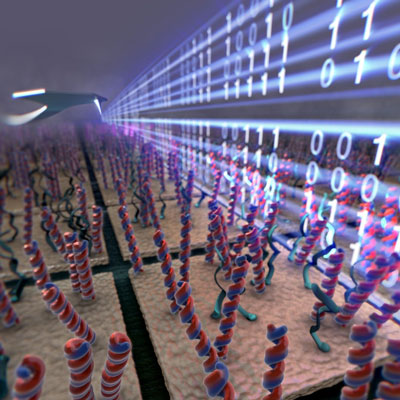
| A new type of nanoscale sensor feels the stiffness of DNA molecules on a microarray to distinguish the ones that formed the double helix with digital precision. The new technology dramatically improves and simplifies microarray analysis. (Image: Sahin Group, Rowland Institute at Harvard) | |
| He points out that besides the sensitivity, the most important advantage is the ability to eliminate all the biochemical processing of the molecules being detected. In most cases concerning gene expression (the degree with which a certain gene is activated in a cell), the main targets are RNA molecules. Because these molecules are not stable and difficult to amplify using biochemical methods like PCR, the RNA sequence is first converted into DNA (using a biochemical process called reverse transcription) and then amplified, labeled, and finally detected. | |
| In contrast, this novel approach directly detects RNAs, because they also hybridize to the DNA that is put on the surface. This way, all the steps beyond extracting RNA from the biological sample are eliminated. This saves a lot of efforts and potentially improves the accuracy. "Later we noticed it was extremely crucial for a recently emerged field in genetics" says Sahin. | |
| Recently, an important biological role of a new class of RNA molecules has been discovered. These molecules are called microRNAs and they regulate the expression level of other genes. They are ?the master regulators?. This newly discovered layer of control inside the cell brought new perspectives to many of problems that biologists are struggling with. They are rather short (about 22 nucleotides long), and their detection is even more difficult because they are not compatible with conventional biochemical processes. | |
| As Sahin notes, "because we directly detect hybridizing RNAs, detection of microRNAs is no different for us and we eliminate all the biochemistry involved in microRNA detection. In our paper published in Nature, we take different cancer tissues and analyze the expression levels of certain microRNAs. This is a miniaturized version of a diagnostic application put forward by a recent publication in Nature Biotechnology by another team, where the primary origin of a tumor is found by comparing expression levels of certain microRNAs. This can be extremely helpful when a pathologist cannot determine the tissue origin of a metastatic tumor. This is not rare. Knowing the tissue origin allows the doctors to prescribe a better treatment plan." | |
| With this new detection platform, one can envision comprehensive genetic analysis being carried out at the doctor's office. More accurate results, early testing and diagnostics are befits that come from the improved sensitivity. Contagious diseases, like influenza or H1N1, have their genetic fingerprints that could be targeted with Sahin's sensor with simplified testing procedures. | |
| "While medical applications are important, what excites me most is the potential of novel scientific insights into cellular processes," says Sahin. "Because our method has the capacity to analyze genetic content at the single cell level, new experiments looking at single cells from a systems biology perspective can be enabled." | |
| Bottom line is that this detection technology exploiting DNA nanomechanics combines the possibility of working with extremely small amounts of genetic material with substantial simplifications and cost reduction in genetic analysis. | |
| Although the researchers say that they are still working on improving throughput in order to enable other biological applications, the scale of improvement is astonishing: For their Nature paper the team has recorded about 200 million mechanical measurements (these are called force-distance curves in the fields of AFM or optical tweezers). According to Sahin, this number is more than what so far has been reported in the entire academic literature! | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
