| Posted: Feb 16, 2010 | |
Another step towards inexpensive hydrogen production from sunlight |
|
| (Nanowerk Spotlight) Companies developing hydrogen-powered technologies like to wrap themselves in the green glow of enviromentally friendly technology that will save the planet. While hydrogen fuel indeed is a clean energy carrier, the source of that hydrogen often is as dirty as it gets. The problem is that you can't dig a well to tap hydrogen, but hydrogen has to be produced, and that can be done using a variety of resources. | |
| The dirtiest method – at least until highly efficient carbon capture and sequestration technologies are developed – is the gasification of coal (read more: "Nanotechnology could clean up the hydrogen car's dirty little secret"). The cleanest by far would be renewable energy electrolysis: using renewable energy technologies such as wind, solar, geo- and hydrothermal power to split water into hydrogen and oxygen. | |
| Especially solar-powered splitting of water promises an attractive, clean energy source and numerous research projects around the world are working on making this process sufficiently efficient – reducing the systems' cost and extending their lifetimes – to be able to compete with dirty carbon fuels on an industrial scale. | |
| Artificial photosynthesis, using solar energy to split water generating hydrogen and oxygen, can offer a clean and portable source of energy supply as durable as the sunlight. It takes about 2.5 volts to break a single water molecule down into oxygen along with negatively charged electrons and positively charged protons. It is the extraction and separation of these oppositely charged electrons and protons from water molecules that provides the electric power. | |
| Natural photosynthesis uses chlorophyll to absorbe visible light and many solar hydrogen cells are imitating this process by using light-sensitive organic dye molecules as light absorbers and then transfer the absorbed energy to a catalyst that reduces protons to hydrogen. | |
| Researchers in the UK have now shown that an inexpensive and environmentally benign inorganic light harvesting nanocrystal array can be combined with a low-cost electrocatalyst that contains abundant elements to fabricate an inexpensive and stable system for photoelectrochemical hydrogen production. | |
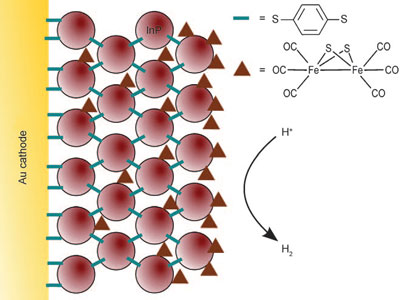 |
|
| Cross-section of a indium phosphide (InP) nanocrystal-modified gold electrode with adsorbed/intercalated subsite analogue. (Reprinted with permission from Wiley-VCH Verlag) | |
| "We have discovered a robust and efficient system for the photoelectrochemical production of hydrogen" Thomas Nann tells Nanowerk. "Unlike most other state-of-the-art approaches, our system does not rely on excited states of organic dyes. Quantum dots have a much larger absorption cross-section and are not prone to photobleaching. This is probably the reason for the increased performance of our system." | |
| Nann, a professor of chemistry and Chair in Nanoscale Science at the University of East Anglia in the UK, together with colleagues from the university's Energy Materials Laboratory, have published their findings in the February 5, 2010 online issue of Angewandte Chemie International Edition ("Water Splitting by Visible Light: A Nanophotocathode for Hydrogen Production"). | |
| The UK team constructed their device by first building-up a cross-linked indium phosphide quantum dot array layer by layer and then incorporating an iron–sulfur electrocatalyst. Underlying the nanocrystals is a layer of gold substrate. | |
| "With our system we were able to achieve a photoelectrochemical efficiency of more than 60%, which is a major breakthrough in this field" says Nann. Note that 60% is not the photoefficiency but the amount of excited electrons (photoelectrons) in the system that are actually used for proton reduction. | |
| The researchers demonstrated the robustness of their system by sustaining a photocurrent for at least one hour without degradation at a bias potential of 400 mV. | |
| Nann points out that these results are still preliminary and represent a proof-of-concept. "The ultimate challenge will be to realize a system which is capable to produce hydrogen in an economically and ecologically viable way." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
