| Posted: Feb 23, 2007 | |
Molecular self-assembly of nanowires |
|
| (Nanowerk Spotlight) Strong and highly directional hydrogen-bonding networks are of fundamental importance in nature. Their efficiency in assisting electron-transfer processes makes them increasingly appealing for technological application inspired by biomimetic principles, i.e. the application of methods and systems found in nature to the study and design of engineering systems and modern technology. Attempting to move from microelectronics to nanoelectronics, engineers are faced with the growing difficulty of manufacturing ever tinier devices with top-down engineering approaches. They are therefore looking at possible ways for bottom-up engineering approaches with the goal of achieving the holy grail of nanotechnology – molecular self-assembly. For some time now researchers have been able to design molecules in such a way that they attach themselves to each other in alternating order, and under certain circumstances – for example on surfaces – create chains. Unfortunately the chains are not very long, because all surfaces, even extremely smooth ones, show unevenness at the atomic level. Step edges, although only a few atomic layers high, represent insurmountable hurdles to the self-assembly process, and since they are distributed randomly over the surface, the molecules form themselves into very irregular patterns. Overcoming this problem, researchers were recently able to formulate two organic molecules in such a way that they organized themselves spontaneously into long parallel chains (nanowires) on a specially prepared gold surface. Selective self-assembly on surfaces and the fundamental processes which control this phenomenon are, however, not only critical in the area of molecular electronics but also in heterogeneous catalysis – a process used in automotive catalytic converters – and in sensor technologies. | |
| "We have demonstrated that the formation of specific 1D hydrogen-bonding networks and their long-range growth can be controlled by a rational design of complementary building blocks for 1D multitopic hydrogen bonding and the choice of an appropriate template surface," Dr. Roman Fasel explains to Nanowerk. "The resulting superlattice of single- and double-row heteromolecular wires exhibits an almost constant wire-to-wire separation over extended surface areas." | |
| Faced with the problem of overcoming the hurdle of step edges in the self-assembly process, Fasel and collaborators at the nanotech@surfaces group at Empa in Switzerland, and colleagues from Ecole Polytechnique Fédérale de Lausanne, the Max-Planck-Institute for Polymer Research, and the University of British Columbia, asked themselves what would happen if all the steps on a surface were arranged parallel to each other like an immense miniature staircase. Theoretically, the molecular chains - which assemble preferentially along step edges - should organize themselves into a long, parallel lattice. | |
| To test this hypothesis, they suitably prepared the surface of a gold single-crystal. After numerous cycles of argon ion bombardment – a cleaning step which removes minute traces of contamination from the gold surface – and subsequent heating the scientists achieved their goal. The surface of the crystal now consisted of a flight of stairs of countless parallel steps all of the same height, namely one gold atomic layer or 0.24 nanometers, with each step separated from the next by a constant distance of 5.8 nanometers. | |
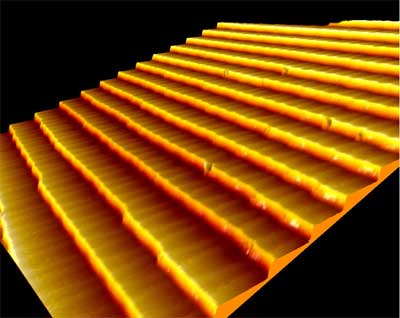 |
Under the scanning tunneling microscope: regular ordering of steps, 0.24 nanometers high and 5.8 nanometers wide, on a specially prepare gold surface (image area 100 x 100 nanometers). (Image: Marta Cañas-Ventura/Empa) |
| "Now all that was necessary for us to do was to evaporate the constituent components of the nanochains onto the gold surface under ultra-high vacuum" says Fasel. "One of the organic building block molecules we used had been specially synthesized by colleagues at the Max-Planck-Institute for Polymer Research and was shaped so as to match the second component. Thus both ends of each molecule possessed structures which fitted together perfectly with the partner molecule, the joint being held together by hydrogen bonds." | |
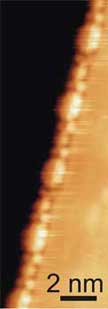 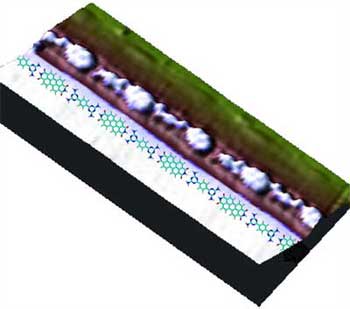 |
Scanning tunneling microscope image of a supramolecular chain (left) and a 3-D representation of the molecular chain with a schematic model of the alternating arrangement of building blocks superposed. (Images: Marta Cañas-Ventura/Empa) |
| What the researchers found was that when low concentrations of the two building block molecules were used, a single chain was formed at each step edge. When higher concentrations were used a double chain was deposited. The double chains, with defect-free zones of about 30 nanometers length, in fact showed significantly better ordering than the single chains, "probably because two chains stabilize each other," according to Fasel. | |
| The complete picture showed a kind of lattice over the whole surface of the gold crystal, with countless evenly spaced nanochains running across it. | |
| "Our study is a proof-of-principle investigation" Fasel points out. "We have been able to show that it is fundamentally possible to grow supramolecular chains laid out in parallel over a surface – and that too over relatively large distances." | |
| The self-assembling supramolecular chains do have one drawback though. They are not suitable as conductors for use in molecular electronics, because on the one hand they are in contact with a metallic substrate – gold – and on the other they show a rather low electrical conductivity. | |
| The Empa team is therefore working intensively on methods, and also other classes of molecules, for creating supramolecular chains on insulating substrates which are more suited for conducting electrical current. In addition "switchable" molecules, which might one day take on the role of transistors in electronic circuits made out of self-assembling molecules, are of particular interest. | |
| The long term aims of the research is, according to Fasel, to understand and control the process of molecular self-assembly in order to develop applications on the nanometer scale not just in the laboratory, but also for use in industrial production. | |
| A paper on this research, titled "Self-Assembly of Periodic Bicomponent Wires and Ribbons" was published in the February 2, 2007 online edition of Angewandte Chemie International Edition. | |
| We ran a Nanowerk Spotlight ("Highly ordered nanostructures through molecular self-assembly") just last December on Fasel's previous work on guided self-assembly of molecules on specially prepared surfaces. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
