| Posted: Mar 01, 2007 | |
Single fluorescent nanodiamonds as cellular biomarkers |
|
| (Nanowerk Spotlight) One of the key avenues to understanding how biological systems function at the molecular level is to probe biomolecules individually and observe how they interact with each other directly in vivo. Laser-induced fluorescence is a technique widely adopted for this purpose owing to its ultrahigh sensitivity and capabilities of performing multiple-probe detection. | |
| However, in applying this technique to imaging and tracking a single molecule or particle in a biological cell, progress is often hampered by the presence of ubiquitous endogenous components such as flavins and collagens that produce high fluorescence background signals. These biomolecules typically absorb light at wavelengths in the range of 300–500 nm and fluoresce at 400–550 nm. To avoid such interference, a good biological fluorescent probe should absorb light at a wavelength longer than 500 nm and emit light at a wavelength longer than 600 nm, at which the emission has a long penetration depth through cells and tissues. | |
| Organic dyes and fluorescent proteins are two types of molecules often used to meet such a requirement; however, the detrimental photophysical properties of these molecules, such as photobleaching and blinking, inevitably restrict their applications for long-term in vitro or in vivo observations. Fluorescent semiconductor nanocrystals (or quantum dots), on the other hand, hold a number of advantageous features including high photobleaching thresholds and broad excitation but narrow emission spectra well suited for multicolor labeling and detection. Unfortunately, most quantum dots are toxic, and hence reduction of cytotoxicity and human toxicity through surface modification plays a pivotal role in their successful application to in vivo labeling, imaging, and diagnosis. | |
| Researchers in Taiwan have demonstrated that nanodiamond particles possess several unique features, including facile surface modification, long-term photostability, and no fluorescence blinking, that makes their detection and long-term tracking in living cells not only possible but practical. | |
| Developed originally for surface finishing industry, the diamond nanoparticle, interestingly, is now finding new and far-reaching applications in modern biomedical science and biotechnologies. | |
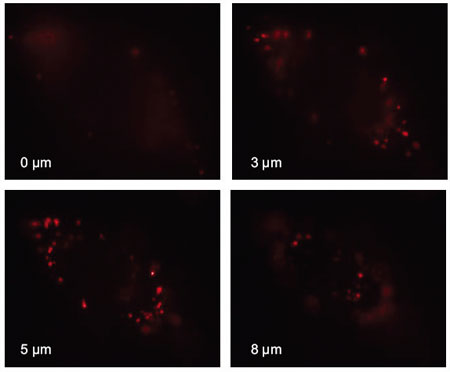 |
Vertical cross-section scans (0-8 microns) of the wide-field epifluorescence image of a single HeLa cell after fluorescent nanodiamond (FND) uptake. No FNDs were found in the central region where the nucleus resided. These particles reappeared when the vertical scan passed across this region. As evidenced by the images taken at 3-5 microns, most of the uptaken FND particles are distributed in the cytoplasm and do not enter the nucleus of the cell. (Reprinted with permission from the National Academy of Sciences) |
| "Our recent findings indicate that fluorescent nanodiamond is an ideal candidate for replacing organic dyes, fluorescent proteins, or semiconductor quantum dots in many biological, particularly, in vivo, applications" Dr. >Huan-Cheng Chang and Dr. Wunshain Fann explain to Nanowerk. "We not only demonstrated that the fluorescence properties of the individual nanodiamonds are not altered even after severe surface modification with strong oxidative acid treatment, we also found that the shelf lifetime of the nanomaterial is very long and the photo-excited fluorescence appears to last forever. In addition, the single nanodiamond does not exhibit blinking behavior." | |
| Fann and Chang, research fellows in the Biophysics and Bioanalytical Technology Group at the Institute of Atomic and Molecular Sciences (IAMS) at Academia Sinica in Taipei, Taiwan, are the corresponding authors of a recent paper on these findings, published in the January 9, 2007 online edition of PNAS ("Characterization and application of single fluorescent nanodiamonds as cellular biomarkers"). | |
| This research is a continuation of the group's previous work published on JACS in 2005 ("Bright Fluorescent Nanodiamonds: No Photobleaching and Low Cytotoxicity"). Then, the researchers already suggested nanodiamonds as an alternative to semiconductor quantum dots. They pointed out that the surface of nanodiamonds can be easily functionalized with carboxyl groups and their derivatives for specific or nonspecific binding with nucleic acids and proteins. Such a unique characteristic opens many opportunities for both in vitro and in vivo applications of fluorescent nanodiamonds. One such example consists of coating carboxylated nanodiamonds with polyL-lysines to facilitate binding of the particles nonspecifically with DNA through electrostatic interactions. | |
| "We believe that this is the first time that bright fluorescent carbon nanoparticles (or "carbon dots" as named by some researchers) were developed and used as a biological probe without fluorescence dye labeling (unlike that of carbon nanotubes) for in vivo applications" says Fann, who heads the Optical Physics Laboratory at IAMS. | |
| "Color-center-containing nanodiamonds, as prepared in our laboratories, are the only carbon-based nanoparticles we know of to date that can produce fluorescence brightness comparable to that of quantum dots" adds Chang. | |
| Fann's and Chang's work was motivated by the awareness that semiconductor quantum dots are toxic and therefore not really well suited for biological applications. Most researchers acknowledge the need for finding better, particularly environmentally friendly, fluorescent probes. Carbon nanoparticles are obvious choices. | |
| The specific problem that could be solved with these nanodiamonds includes tracking of a single fluorescent nanodiamond particle carrying target molecules during the course of cell differentiation and development in three dimensions. These processes usually take hours to complete and cannot be monitored continuously using conventional fluorescent probes. How a virus affects a cell is another problem that can be solved with fluorescent nanodiamonds. | |
| The most important potential biological applications of fluorescent nanodiamonds include (1) biomolecular labeling, (2) cellular imaging, (3) tumor targeting, (4) single particle tracking, (5) long-term in vivo monitoring. | |
| Fann and Chang point out that the future direction of their research is to find real-world biological applications for fluorescent nanodiamonds. "A big step toward their goal will be making these bright diamond beacons commercially available to all researchers who have ideas and would like to try it out in their own laboratories" they say. | |
|
Nevertheless, there are many challenges the researchers still are facing in their current and future research. To name three of them, they list:
|
|
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
