| Posted: Apr 08, 2010 | |
Nanotechnology water sensor for highly sensitive detection of cyanide |
|
| (Nanowerk Spotlight) Nanotechnology can play an important role in water treatment and an active emerging area of research is the development of novel nanomaterials with increased affinity, capacity, and selectivity for heavy metals and other contaminants. The benefits from use of nanomaterials may derive from their enhanced reactivity, surface area and sequestration characteristics (see for instance "Smart capsules for water treatment with recyclable carbon nanotube cores"). | |
| In addition to heavy-metal ions such as mercury or lead, toxic anions like cyanide are of concern to environmental managers. Whereas toxic metals induce diseases by accumulating in the body, cyanide can directly cause death in as short a period as minutes by directly affecting the central nervous system. | |
| Researchers in China have now developed a relatively simple, cost-effective, environmentally friendly and yet highly sensitive cyanide sensor to test water samples. | |
| "Compared with many current optical chemosensors for cyanide, our gold-nanocluster-based sensing system is very simple in fabrication and operation, without requiring complex organic synthesis and complicated instruments," Lehui Lu explains to Nanowerk. "Besides, the unique Elsner reaction between cyanide and gold atoms in gold nanoclusters surmounts interference from other anions. Most importantly, this sensing system can work directly in pure aqueous solution, thus enabling monitoring of practical water samples." | |
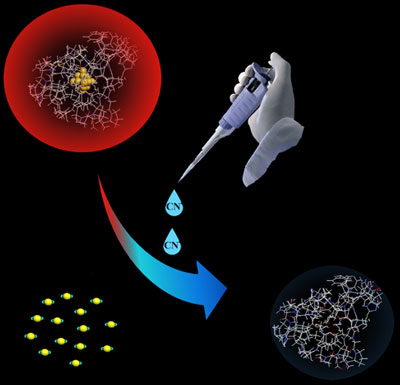 |
|
| Schematic representation of the gold nanocluster--based sensor for cyanide. An aqueous solution of the gold nanoclusters exhibits a bright-red fluorescence under UV light. After addition of cyanide, only a very-weak blue fluorescence can be observed. (Image: Dr. Lu, CAS) | |
| Lu, a professor in the State Key Laboratory of Electroanalytical Chemistry at the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, and his team have demonstrated their sensing system on several real water samples spiked with cyanide. They found that the lowest concentration their sensor allows to quantify is approximately 14 times lower than the World Health Organization's guideline value for cyanide in drinking water – which is 0.07mg per liter. | |
| The fluorescent sensor, as reported in a recent edition of Advanced Functional Materials ("Gold-Nanocluster-Based Fluorescent Sensors for Highly Sensitive and Selective Detection of Cyanide in Water"), is based upon the cyanide etching-triggered fluorescence quenching of the gold nanoclusters. Lu points out that it can work directly in aqueous solution and does not require any toxic organic reagent as an assistant solvent. | |
| Gold nanoclusters are a novel type of fluorescence materials developed in recent years. Compared with traditional dyes or other organic chromophore, the fluorescence of gold nanoclusters is relatively stable, and unlike some semiconductor materials, gold nanoclusters stabilized with bovine serum albumin (BSA), as in this work, are rather low toxic. Most importantly, the fluorescence intensity is highly size-dependent, making it very suitable as a nanoscale sensing system. | |
| In their work, the Chinese team prepared BSA-stabilized gold nanoclusters that consisted only of several to tens of atoms, making them smaller than 1 nanometer in size. | |
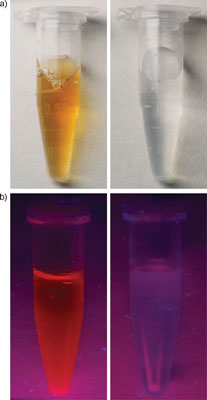 |
|
| a) Aqueous solution of gold nanoclusters before (left) and after (right) addition of 5x10-3 Μ cyanide. b) Fluorescence of the aqueous solution of gold nanoclusters before and after addition of 5x10-3 Μ cyanide under UV light at 365 nm. (Reprinted with permission from Wiley-Verlag VCH) | |
| "The aqueous solution of the gold nanoclusters was deep brown in color and exhibited a bright-red fluorescence under 365nm UV light," explains Lu. "After addition of cyanide, the original deep-brown color of the gold nanoclusters became colorless, and a very-weak blue fluorescence was observed under 365nm UV light. The corresponding fluorescence spectrum showed that the maximum emission peak at 640 nm, characteristic of the gold nanoclusters, disappeared." | |
| One particular advantage of this gold nanocluster sensor is that it is highly selective towards cyanide over other anions. One problem with current optical chemosensors for cyanide is that they are easily disturbed by other anions. To investigate whether our system is specific for cyanide, Lu's team measured the fluorescence response of their cyanide sensor with eighteen common anions under the same conditions. | |
| "We found that only cyanide could induce a drastic decrease in the fluorescence intensity, whereas, no obvious fluorescence changes were observed in the presence of other anions," says Lu. "The tolerance concentrations of these anions when detecting cyanide using gold nanoclusters were at least 20 times the cyanide anion concentration. | |
| To test their sensor under real-world conditions, the researchers used water samples including local groundwater, tap water, pond water, and water from a nearby lake and laced them with cyanide. The sensor gave no obvious fluorescence response to the plain water samples but all cyanide water samples led to a significant decrease in the fluorescence intensity of the sensing system. Interestingly, the change of fluorescence intensity was almost identical for all water samples, a further indication that such a sensing system is highly selective toward cyanide over other compositions in real water samples. | |
| "These striking properties substantially give this simple, cost-effective sensing system a great potential for reliable detection of cyanide in food, soil, water and biological samples," concludes Lu. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
