| Posted: May 03, 2010 | |
Nanotechnology probe taps into algae cell and saps electrical energy |
|
| (Nanowerk Spotlight) Photosynthesis is the process by which plants convert solar energy into chemical energy. In the presence of visible light, carbon dioxide and water are transformed into glucose and oxygen during a complex series of chemical reactions. The solar energy, now converted to chemical energy, is stored in the bonds of sugars and polysaccharides that plants use for food. | |
| The process takes place in chloroplasts, the cellular powerhouses that make sugars and give leaves and algae their green color. In the chloroplasts, water is split into oxygen, protons and electrons. Sunlight penetrates the chloroplast and zaps the electrons to a high energy level, and a protein promptly grabs them. The electrons are passed down a series of proteins, which successively capture more and more of the electrons' energy to synthesize sugars until all the electrons' energy is spent. | |
| There are several ways to extract the energy from the photosynthetic conversion process. The dirty, old way that our society has been relying on so far is by simply burning fossil fuels such as coal, natural gas and oil. More recent efforts have focused on using biomass to produce biofuels – quite controversial, too, although there appear to be interesting nanotechnology enabled production methods (see: Ethanol production inside carbon nanotubes and Nanotechnology's role in next generation biofuel production). | |
| An intriguing novel approach has now been demonstrated by researchers at Stanford and Yonsei Universities. They have inserted ultrasharp gold nanoelectrodes (custom-made, AFM- compatible, nanoscale electrochemical probes that were shaped in the form of a flat, sharp needle with an aspect ratio of 10 and tip diameter of less than 30 nm) into living algae cells and extracted electrons, thereby harnessing an – albeit very tiny – electrical current. This is electricity production that doesn't release carbon into the atmosphere. The only byproducts of photosynthesis are protons and oxygen. | |
| The ultrasharp nanoprobes associated with the system allowed for penetration of the cell or chloroplast membranes using relatively low force and without disrupting the integrity of the cell (the cell membrane seals around the hydrophobic insulating material of the electrode). | |
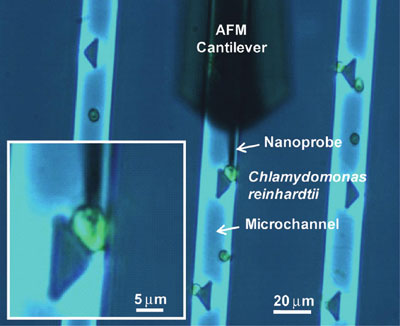 |
|
| Microfluidic immobilization platform for visualization and high throughput electrochemical measurements. Penetration of a cell by the AFM-navigated nanoelectrode. (Reprinted with permission from American Chemical Society) | |
| Reporting their findings in a recent paper in Nano Letters ("Direct Extraction of Photosynthetic Electrons from Single Algal Cells by Nanoprobing System"), the team, led by Fritz B. Prinz demonstrates the aerobic extraction of photosynthetic high-energy electrons, both with and without mediators, from the single-celled alga Chlamydomonas reinhardtii. The results demonstrate the feasibility of collecting high-energy electrons in steps of the photosynthetic electron transport chain and prior to the downstream processes associated with energy loss. | |
| The authors (Seoung-Jai Bai, Tibor Fabian, Rainer J. Fasching, Zubin Huang and Joong Sun Park, all researchers in the Rapid Prototyping Laboratory for Energy and Biology at Stanford University; and Jeffrey Moseley and Arthur Grossman, researchers in the Department of Plant Biology at the Carnegie Institution and the Department of Biology at Stanford) say that this approach potentially reduces energy losses associated with the multistep transformation of solar energy into products used for the production of biodiesel and bioelectricity. | |
| In addition, the system allows direct monitoring of specific charge transfer reactions in live cells, leading to broad applications for investigating developmental processes and the responses of cells and organelles to light and chemical stimuli. | |
| WonHyoung Ryu, first author of the paper and a member of Prinz's group during this work (now a professor at Yonsei University in Seoul), says that the team was able to draw from each cell just a little bit over one picoampere – which is equivalent to a yield of 0.6 milliamps per square centimeter. By contrast, some silicon solar cells have a current density of 35 milliamps per square centimeter. Another way of looking at how tiny this amount of electricity is: you would need a trillion cells photosynthesizing for one hour just to equal the amount of energy stored in a AA battery. | |
| In addition, the cells die after an hour. Ryu said tiny leaks in the membrane around the electrode could be killing the cells, or they may be dying because they're losing out on energy they would normally use for their own life processes. | |
| "One of the next steps would be to tweak the design of the electrode to extend the life of the cell," he says. "Other possible next steps to make this a practical system would be to use a plant with larger chloroplasts for a larger collecting area, and a bigger electrode that could capture more electrons. With a longer-lived plant and better collecting ability, we could scale up the process." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
