| Posted: Aug 09, 2010 | |
Investigating the impact of carbon nanotubes on male reproductive health |
|
| (Nanowerk Spotlight) With fully conclusive findings about the toxicity of carbon nanotubes (CNTs) still up in the air (see "The ongoing challenge of determining carbon nanotube toxicity"), research on biomedical applications of CNTs is pushing full steam ahead. Adding to the list of potential concerns, a recent nanotoxicology study by a U.S.-Chinese research team looked into the impact of carbon nanotubes on male reproductive health. | |
| The translocation and biodistribution of nanoparticles are key factors in their toxicity evaluation in vivo. Although other nanoparticles such as gold and magnetic nanoparticles have been reported to enter testes in small quantities, it had not been established whether CNTs could enter or accumulate in the testis. | |
| "Our pilot study investigated the effects of intravenous injection of single and multiple doses of water-soluble multiwalled carbon nanotubes on the reproductive systems of male mice," Bing Yan, Director, High-Throughput Analytical Chemistry Facility at St. Jude Children's Research Hospital in Memphis, TN, tells Nanowerk. "Although our study showed that carbon nanotubes have minor effects on the male reproductive system in mice, oxidative stress and the alterations in the testes raise concerns because it is possible that these materials may accumulate at higher quantities over a longer period and may have adverse effects on male fertility." | |
| Reporting their findings in the August 8, 2010 online issue of Nature Nanotechnology ("Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility"), Yan and his colleagues together with collaborators from Shadong University in Jinan, China, conclude that carbon nanotubes, when used at their doses and schedule, can cause reversible testis damage and reactive oxygen species (ROS) generation without changing the hormonal levels, sperm health, and male mice fertility. | |
| To mimic the potential biomedical applications of the carbon nanotubes in terms of administration method and dose, the team intravenously injected the nanotube suspension through the tail vein into healthy adult male mice. The nanotubes were administered either as a single dose of 5 mg per kg or in five doses over 13 days at 5 mg per kg per dose. They then conducted reproductive toxicologic assessments on days 15, 60 and 90. | |
| "Within 24 hours we found nanotubes in the testis, and accumulation resulted in oxidative stress and tissue damage" says Yan. "However, the damage was reversed after two months, and we observed no effects on mating, fertility, delivery or fetus viability under our experimental conditions. Sex hormones and sperm were unaffected by the nanotubes throughout the 90-day period, and treated mice continued mating with healthy female mice to produce healthy offspring." | |
| Yan explains that the team examined the accumulation of 64Cu-labelled carboxylated carbon nanotubes in the testes after a single dose. What they found were approximately 41, 61 and 151 ng of nanotubes in the testes 10 minutes, 60 minutes and 24 hours after injection, respectively. | |
| "Although the relative amount of nanotubes in the testes was small, the increasing trend suggests that with five repeated doses, more nanotubes would be expected to accumulate there" he says. | |
 |
|
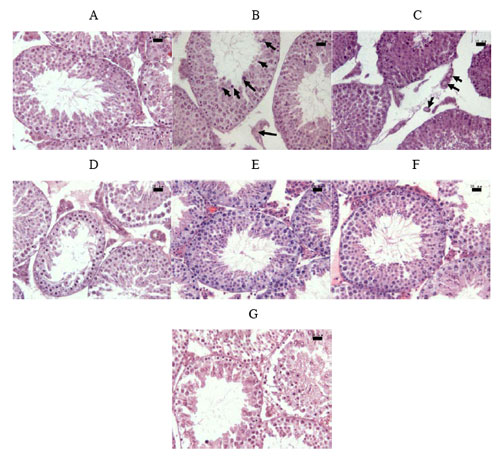
| Pathologic examination of testes from mice treated with MWCNTs and PBS. Testicular sections of the MWCNT-COOH treated mice by Administration Scheme 1 (A) showing normal seminiferous tubules with unaffected spermatogenic cells. Testicular section of MWCNT-COOH treated mice by Exposure Scheme 2 for 15 days (B) showing necrotic and degenerative changes in the form of multinucleated giant cells. (Arrows). (C) Vasodilatation and hyperemia (Arrows) were also found in testicular section of MWCNT-COOH treated mice by Exposure Scheme 2 for 15 days. Testicular section of MWCNT-NH2 treated mice by Exposure Scheme 2 for 15 days (D), 60 days (E) and 90 days (F) showing the similar histological effects as MWCNT-COOH treated mice with less severity. Testicular section of MWCNT-COOH treated mouse by Exposure Scheme 2 for 15 days (G) showing very severe damage to the seminiferous tubules. (There is only one mouse in this group showed this injury). Scale bars, 20 µm. (Reprinted with permission from Nature Publishing Group) | |
| Yan points out that, after nanotube injection, throughout the entire experimental period, none of the mice from any group showed stress or symptoms of abnormality. But he notes that the accumulation of nanotubes in the testes raised the question of whether they could adversely affect Sertoli cells and seminiferous tubules. The testes from mice treated with five doses of carboxylated nanotubes were characterized by partially damaged seminiferous tubules, a significant reduction in the thickness of the germinative layer, and a reduction in the number of spermatogonia. Histologic studies also showed a partial disappearance or vacuolization of Sertoli cells. | |
| "The initial histologic alterations and the increase in oxidative stress in the testes indicate that nanotubes may harm the male reproductive system" says Yan. "However, in a dosing schedule similar to typical biomedical applications, the extent of damage in the testes was much less than the damage caused by other toxicants." | |
| This work by Yan and his collaborators systematically investigates male reproductivity toxicity of carbon nanotubes, broadens our understanding on the toxicological profiles of nanomaterials, and paves the way for the safe development of numerous medicinal applications of carbon nanotubes. | |
| This work defines a possible range of safe doses if carbon nanotubes are to be used in medicine. Although carbon nanotubes induced initial pathologic alterations in the testes of mice, these alterations showed signs of recovery over time. The lack of adverse effects on the quality and quantity of the sperm further support this observation. | |
| However, the results also indicate that higher doses or more repeated dosing schedules will likely cause severe damages in male reproductive systems. | |
| "Considering the highly diverse structures and properties of nanomaterials and the multiple ways in which human exposure to nanomaterials can occur, further studies on the reproductive toxicity of nanomaterials, particularly following long-term and early life exposure, are urgently needed" concludes Yan. | |
| He also points out that more work needs to be conducted in nano-reproductive toxicology. "The future directions for this field includes: 1) nano-female reproductive toxicological study; 2) nano-developmental toxicological study; 3) reproductive toxicology with long-term exposure; 4) reproductive toxicological study after early life exposure of nanomaterials" he says. | |
| "The decline of human fertility is a result of very complicated and prolonged processes and complicated factors. As we are going into a society full of nanomaterials, how to mimic the real exposure of nanomaterials to human beings in the lab is key. Furthermore, how to evaluate the combined effects with other pollutants and factors and how to realize a quicker evaluation of nano-reproductive toxicity are all challenges facing our future research in this area." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
