| Posted: Aug 10, 2010 | |
Effective protein therapy with nanochannel membranes |
|
| (Nanowerk Spotlight) Delivering healthy proteins directly into human cells to replace malfunctioning proteins is considered one of the most direct and safe approaches for treating diseases. Controlled and long-term protein drug delivery has also been considered as one of the most promising biomedical applications of nanotechnology. | |
| So far, though, the effectiveness of protein therapy has been limited by low delivery efficiency and the poor stability of proteins, which are frequently broken down and digested by cells' protease enzymes before they reach their intended target. This not only makes the drugs ineffective, it can also cause unpredictable side effects such as inflammation, toxicity, and immune responses. | |
| The most widely used method for delivery of protein drugs has been to incorporate them into biodegradable microparticles. This approach was withdrawn from the market due to the protein denaturation by hydrophobic interaction and/or harsh acidic microenvironments caused by the degradation of polymers inside the body. | |
| "The best way for the delivery of protein drugs without denaturation might be possible by exploiting the passive diffusion through a membrane without physical and chemical stresses," Jin Kon Kim explains to Nanowerk. "This can be achieved when pore sizes in a membrane are controlled to satisfy the single-file diffusion (SFD) condition of protein drugs." | |
| Kim, a professor in the Department of Chemical Engineering, and director of National Creativity Research Initiative Program for Block Copolymer Self Assembly at Pohang University of Science Technology (POSTECH) in South Korea, and his team have developed a new drug delivery device with nanoporous membrane based on block copolymer self-assembly for the constant and long-term release of protein drugs without denaturation. | |
| "By tuning the pore size of membrane depending on the hydrodynamic diameter of target drugs, we achieved the single file diffusion of protein drugs" says Kim. "Since two or more diffusing molecules could not be allowed to pass through the pores simultaneously, the release rate of a protein drug becomes constant with time irrespective of its concentration in the reservoirs. This phenomenon is analogous to the constant dropping rate of sands through an hour-glass with time." | |
 |
|
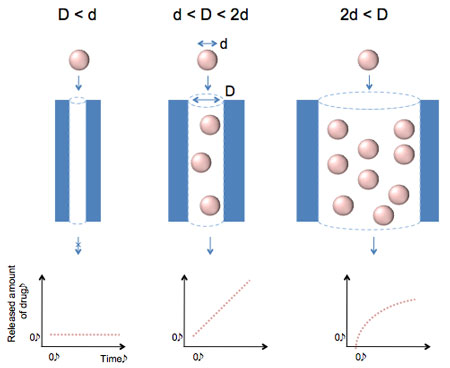
| Release behavior of a protein drugs depending on channel diameters (D) and protein sizes (d). When D is less than d, it could not release at all (left image). When D is much bigger than 2d, a constant release of cannot be achieved due to burst release at initial times. The high initial concentration of the drugs can cause side effects (right image). However, if D is exactly controlled in the range of between d and 2d, the constant release is obtained like dropping sand in hour glass (middle image). This kind of the diffusion is referred to as the single file diffusion. (Image: Dr. Kim, POSTECH) | |
| Reporting their findings in a recent paper in ACS Nano ("Single-File Diffusion of Protein Drugs through Cylindrical Nanochannels"), the POSTECH researchers are first to demonstrate single-file diffusion of protein drugs in in vivo experiments. | |
| They successfully demonstrated the long-term controlled release of protein drugs by the SFD up to two months using the drug delivery device with cylindrical block copolymer (BCP) nanochannels. | |
| "According to the hydrodynamic diameter of a target protein drug, we precisely controlled the pore size down to 6 nm by gold deposition," explains Kim. "We were also able to control the release rate of protein drugs by changing the length of BCP nanochannels and the thickness of the gold deposition layer." | |
| The precise control of the pore size was made possible by depositing gold on top of the nanoporous polymer membrane as well as on the pore walls as well. Thus, the pore size decreased with increasing the thickness of the gold deposition layer on the membrane. For instance, at a gold deposition layer with 7 nm thickness, the average diameter of nanopores became ca. 10 nm. With further increase of gold layer to 11 nm thickness, the diameter of the pores at the top of the membrane was reduced to 6 nm. | |
| Although the pore size also decreased by the deposition of other metals, the researchers chose gold because of its good biocompatibility and excellent adhesion with the membrane. | |
| Kim and his team believe that, due to facile and cost-effective fabrication processes, their drug delivery device with cylindrical BCP nanochannels could be successfully exploited for long-term constant delivery of protein drugs without denaturation for the treatment of various chronic diseases. | |
| The functionalization of block copolymers or decoration by antibody to the nanochannels is a promising approach for separation of specific materials such as chiral compound. Another interesting application could be water purification (see "Nanoporous Membranes Derived from Block Copolymers: From Drug Delivery to Water Filtration"). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
