| Posted: Sep 24, 2010 | |
Bacteria as environmentally friendly nanoparticle factories |
|
| (Nanowerk Spotlight) In nature, uni- and multicellular organisms are capable of reducing and accumulating metal ions as detoxification and homeostasis mechanisms when exposed to metal ion solutions. Although the exact mechanisms and identities of microbial proteins associated for metal nanoparticle synthesis are not clear, two cysteine-rich, heavy metal-binding biomolecules, phytochelatin and metallothionein have been relatively well characterized. | |
| Phytochelatins are oligo-glutathione peptides of varying sizes that are synthesized by the protein phytochelatin synthase and that can form metal complexes with cadmium, copper, silver, lead and mercury, while metallothioneins are gene-encoded proteins capable of directly binding metals such as copper, cadmium, and zinc. | |
| This capability of phytochelatin and metallothionein – having different metal binding affinities to various metal ions – has now been employed by researchers for the in vivo biosynthesis of metal nanoparticles by recombinant Escherichia coli. | |
| "The strategy of employing recombinant E. coli expressing metal binding proteins as a nanoparticle factory is generally applicable to the combinatorial synthesis of diverse nanoparticles having a wide range of characteristics, such as optical, electronic, chemical, and magnetic properties" Sang Yup Lee, head of the Metabolic & Biomolecular Engineering National Research Laboratory at KAIST, explains to Nanowerk. "Several physico-chemical processes that have been employed for the synthesis of metal nanoparticles involve processes at high temperatures in organic solvents, which are costly and environmentally unfriendly. Nanoparticles synthesized in recombinant E. coli cells are size-tunable at ambient temperature and possess chemical and optical characteristics comparable, if not identical, to those of chemically-synthesized nanoparticles." | |
| Synthesizing nanoparticles in recombinant E. coli could turn out to be an economically and environmentally friendly alternative to current, not so 'green', industrial processes. | |
 |
|
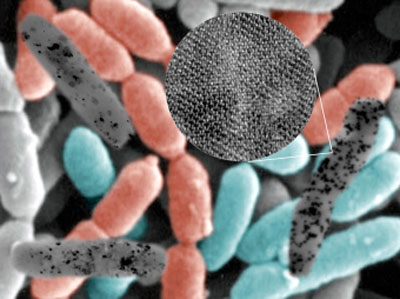
| Recombinant E. coli cells (colored in background) expressing the metallothionein gene and/or phytochelatin synthase gene accumulate metal nanoparticles (black dots in black-and-white cells) upon incubation with corresponding metal ions. (Image: Dr. Lee, KAIST) | |
| In this work, published in the August 18, 2010, online issue of Angewandte Chemie International Edition ("In Vivo Synthesis of Diverse Metal Nanoparticles by Recombinant Escherichia coli"), Lee and his collaborators incubated various metals, including semiconducting (Cd, Se, Zn, Te), alkali-earth (Cs, Sr), magnetic (Fe, Co, Ni, Mn), and noble (Au, Ag) metals and rare-earth fluorides (Pr, Gd), in assorted combinations with the recombinant E. coli cells for the in vivo synthesis of the corresponding metal nanoparticles. | |
| Lee notes that the team was able to control size of the metal nanoparticles by adjusting the concentrations of the supplied metal ions. | |
| "As the high-cell-density culture of E. coli has been well established, the efficient and cost-effective large-scale production of various metal nanoparticles would not be a difficult task" he says. "The engineered E. coli system that we have reported should be broadly applicable to the in vivo synthesis of metal nanoparticles of interest with tailored optical, electronic, chemical, and magnetic properties." | |
| One restriction that became obvious was that E. coli cells show retarded growth profile at metal ion concentrations above 5 mM due to their toxicity. Thus, the size of the metal particles that can be synthesized in vivo is restricted. According to Lee, this can be overcome because cell extracts of E. coli cells expressed metal binding proteins that can be used for the synthesis of metal particles in vitro at a larger scale. | |
| An interesting aspect of this work is that this system could be used to synthesize new types of earth metal nanoparticles – such as CdCs, SrGd and SrPr – that were never synthesized before by chemical methods. | |
| Furthermore, the coexpression of metal binding proteins in recombinant E. coli cells resulted in an enhanced assembly of diverse metal elements into fine-ordered nanoparticles. | |
| The KAIST team is now studying several synthetic strategies for more diverse metal particles by using a microfluidic system and a fusion system with various nanostructures in vitro and in situ as well as in vivo systems. However, for this to be achieved, the metal synthesis mechanism by metal binding proteins first needs be accurately characterized in detail. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
