| Posted: May 09, 2007 | |
Applying a 250-year old discovery to nanotechnology fabrication |
|
| (Nanowerk Spotlight) Back in 1756, the German physicist Johann Gottlob Leidenfrost published a manuscript titled De Aquae Communis Nonnullis Qualitatibus Tractatus ("A Tract About Some Qualities of Common Water") in which he described a phenomenon in which a liquid, in near contact with a mass significantly hotter than its boiling point, produces an insulating vapor layer which keeps that liquid from boiling rapidly. | |
| This effect came to be called the "Leidenfrost Effect" and the associated temperature point the "Leidenfrost Temperature." An everyday example of this can be seen in your own kitchen: sprinkle a drop of water in a hot skillet – if the skillet's temperature is at or above the Leidenfrost Temperature, the water skitters across the metal and takes longer to evaporate than it would in a skillet that is hot, but at a temperature below the Leidenfrost point. | |
| Researchers in Germany have used this effect for a novel, template-free synthesis and patterning method of nanostructures. | |
| While nanoscale self-assembly is one of the core concepts of Nature, scientists are only beginning to scratch the surface of the potential that self-assembly holds for materials engineering. The list of self-assembly fabrication methods for the formation of nanocluster structures seems to get longer and longer but they all feature different timescales, complexities and versatilities. The one thing they usually have in common is the requirement for two steps – the formation of nanoparticles from precursors in the liquid, solid, or gas phases employing either chemical or physical deposition processes and, in a subsequent step, the organization of these particles into useful structures. | |
| Nanoscientists have recognized that template-free approaches of self-organization would be the simplest and most effective way of building nanostructures from the bottom up, as just recently witnessed by IBM's announcement of the first-ever application of a breakthrough self-assembling nanotechnology to conventional chip manufacturing. | |
| "Wet-chemical strategies utilizing fluid mechanics appear to be the simplest and most effective way of template-free, self-assembling nanostructuring" Dr. Rainer Adelung explains to Nanowerk. "However, structuring of nanocluster arrays or wirelike morphologies from a droplet still faces certain challenges." | |
| Adelung, a researcher at the Department of Multicomponent Materials at the University of Kiel, Germany, tells the story of his colleague Mady Elbahri experimenting with new routes for ZnO synthesis and discovering a novel way for a droplet-disposition-based nanostructuring technique. | |
| "Inspired by kitchen experiments with his wife Julia, he discovered that it might be possible to ignore the typical temperature limit of 100°C for water-based synthesis. He found that at temperatures of 250°C, well above the boiling point, it is possible to use water droplets that contain a small amount of chemicals for nanostructuring – thanks to the Leidenfrost effect." | |
| What the researchers in Kiel ultimately developed is a droplet-deposition-based, template-free, and rapid (only a few seconds) approach for fabricating nanostructures without the use of any surfactant. | |
| "Our general setup can be understood as the so-called “anti-Lotus effect”" says Adelung. "The Lotus effect is well known for its removal of dust particles from the surface of a lotus leaf by gathering them into a droplet that is moving over the surface, thus cleaning it. The effect is based on the ability of certain surfaces to form spherical droplets with contact angles near 180° (i.e., superhydrophobic), enabling the incorporation of surface particles as well as a reduction in friction. In contrast to this, our work makes use of an anti-Lotus effect, in which the droplet delivers material while moving over the surface." | |
| It seems that, similar to room temperature droplets on superhydrophobic surfaces, a Leidenfrost droplet can move with highly reduced friction over an arbitrary surface such as plain silicon and deposit nanoparticles. These can be nanoparticles already dispersed in the water droplet or formed in the overheated steam from a mix of chemicals. In this way, reactions leading to nanoparticle synthesis can be performed that do not occur at room temperature (which the Kiel researchers have come to call the "Elbahri synthesis"). | |
| Combining this method with a top down approach enables the formation of regular patterns. A grid with regular pattern of openings is indeed enough to force the self organized structures into a reproducible shape. | |
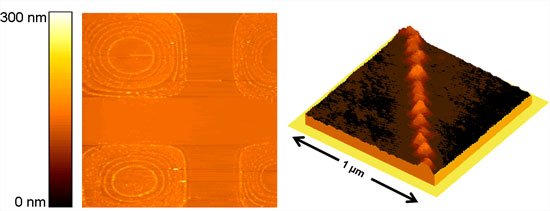 |
The left side of the figure shows such a pattern in a large scale AFM image (baseline is 90 µm). Silver rings that form inside the opening made from silver nitrate are stacked into each other. The larger the diameter is, the smaller the ring cross section down to the nanoscale. The image on the right shows a three dimensional representation from an AFM image of a silver nanowire. (Images: Kahled Hirmas, Rainer Adelung) |
| Adelung points out that a random deposition of a solution drop would be totally ineffective in fathering any technologically useful nanoscale structures, such as arrangements of well-separated patterns prepared from monodisperse particles. | |
| "In order to deposit material from a droplet in an organized manner, a deeper insight into the underlying mechanism of the fluid drop is necessary" he says. | |
| The researchers prepared an extremely dilute solution of the desired material powder in water. Then they gently placed a droplet from this solution on a substrate, such as a silicon wafer, that was maintained at a temperature of 230°C, which is the estimated Leidenfrost temperature for any suspension droplet. They allowed the drop to stay on the hot substrate for approximately 5 seconds before the substrate was tilted ca. 30° to the horizontal to exploit gravity for the propagation of the loaded droplet across the substrate surface. When a water droplet loaded with silver nanoparticles was subjected to this procedure, wires or cluster chains were successfully produced. | |
| "Apart from straight, parallel lines in series, nanoparticles can also be arranged in concentric circles with our Leidenfrost structuring" says Adelung. "We attribute this patterning to a different and interesting phenomenon that also occurs at the Leidenfrost temperature, under slightly modified fluid dynamics, where droplet impact is emphasized. Interfacial, viscous, and capillary forces are the three basic forces governing the behavior of a droplet on a surface. At temperatures below the Leidenfrost temperature, we observed a spreading behavior that results in the formation of a disk like thin film upon impact." | |
| In a next step in their research, Adelung and his colleagues are now planning to measure the electronic properties of the nanostructures for possible sensor applications. | |
| A recent paper in Advanced Materials describes this new nanopatterning method ("Anti-Lotus Effect for Nanostructuring at the Leidenfrost Temperature"). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
