| Posted: Nov 29, 2010 | |
Adding an optoelectronic component to molecular electronics |
|
| (Nanowerk Spotlight) One of the many fascinating concepts in nanotechnology is the vision of molecular electronics where researchers are investigating nanostructured materials to build electronics from individual molecules. If realized, the shift in size from even the most densely packed computer chip today would be staggering. To get a feel for the dimensions involved, just consider that a single drop of water contains more molecules (approx. 1.67x1023) than the billions upon billions of silicon chips ever produced. The nanoelectronics engineers of the future might be capable of using individual molecules to perform the functions in an electronic circuit that are performed by semiconductor devices today. | |
| Molecular electronics aims at the fundamental understanding of charge transport through molecules and is motivated by the vision of molecular circuits to enable miniscule, powerful and energy efficient computers. A research team in Germany has now demonstrated that rigidly wired molecules can emit light under voltage bias. This result is important for fundamental science but it also adds to the molecular electronics vision an optoelectronic component, i.e. the development of optoelectronic components on the basis of single molecules. | |
| "In previous works, electroluminescence from molecules has been observed when contacted by the tip of a scanning tunneling microscope," Ralph Krupke, who heads the Electronic and Optical Properties of Molecular Nanostructures group at at Karlsruhe Institute of Technology (KIT), tells Nanowerk. "In our recent work, our motivation was to form a rigid light-emitting device based on single molecules." | |
| Reporting their findings in the November 28, 2010 issue of Nature Nanotechnology ("Electroluminescence from a single nanotube-molecule-nanotube junction"), the team has realized a rigid solid-state device comprised of single molecules which emit light under voltage bias. | |
| For this purpose, a team led by Krupke and Marcel Mayor, a professor of chemistry at the University of Basel in Switzerland, synthesized a specifically designed rod-like molecule with a fluorescent core and electrodes of carbon nanotubes. | |
| The researchers developed a special technique that allowed them to place this molecule between electrically biased carbon nanotube electrodes. They then used the spectroscopic fingerprint of the molecule as evidence for the molecular electroluminescence. | |
| "Obtaining an electrically triggered luminescence signal from a molecular junction is an endeavour that requires expertise and contributions from experimental physics and synthetic chemistry," explains Krupke. "The major challenge was to integrate a bottom-up object (molecule) into a top-down structure (electrodes) and to have control over the critical dimensions. Moreover the electronic and optical properties of the molecule and the carbon nanotube electrodes had to be tailored such that electron transport and light emission is possible." | |
 |
|
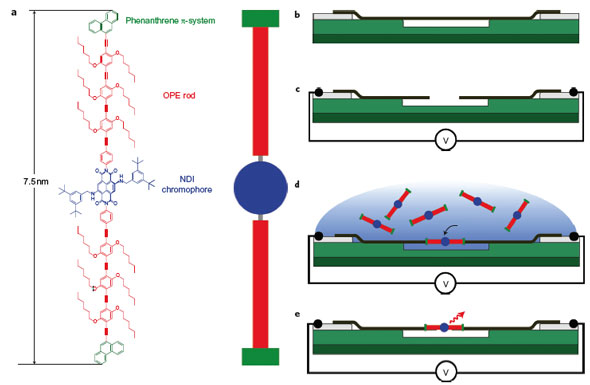
| Chemical structure of the molecule and formation of the nanotube-molecule-nanotube device. a) Structure of the molecule, which consists of a central 2,6-dibenzylamino core-substituted NDI chromophore (blue), two OPE rods (red) and phenanthrene anchor units (green). b–e) Schematics of device fabrication. After dielectrophoretic deposition (b), a metallic nanotube (black) bridges palladium electrodes (grey) and is free-standing above a trench in silicon oxide (green). Electrical breakdown opens a gap in the nanotube (c). Dielectrophoretic deposition of the polarizable molecule from solution into the nanotube gap leads to the formation of a NT–M–NT junction (d). Light emission from the molecule under voltage bias V is detected and analyzed in an optical microscope setup (e, not shown). (Reprinted with permission from Nature Publishing Group) | |
| To that end, two members of the team, Sergio Grunder and Alfred Blaszczyk, synthesized a 7.5nm long rod-like molecule with a chromophore core. Another team member, Christoph W. Marquardt, who also is first author of the Nature Nanotechnology paper, fabricated carbon nanotube electrodes with a tiny gap (<10nm) by a controlled current-induced local oxidation. He was then able to place the molecules from solution in between the nanotube electrodes by dielectrophoresis, an electric field-induced form of self-organization. | |
| Krupke notes that the necessary stability of the nanotube-molecule-nanotube junctions to survive high biasing is provided by special anchor groups at the molecule ends. | |
| "Applying a few volts to the junction causes the molecule to emit light" he says. "In a sensitive optical microscopy setup we were able to detect the light and prove that the light is emitted from the molecule's chromophore core." | |
| In all molecular electronics experiments the question arises of how many molecules contribute to electron transport. Although experimentally the answer is not accessible, Krupke and Mayor argue that the gap formed by nanotube electrodes with a cross-sectional area of ∼1 nm2 can host no more than one to three of the bulky molecules. | |
| The team is currently fabricating junctions with various central chromophores to obtain molecular light-emitting devices that operate at different emission wavelengths. A major challenge will be to tailor molecules with a level energy scheme to reduce non-radiative electron transfer. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
