| Posted: Feb 09, 2011 | |
Fundamental processes involved in nanoparticle synthesis still not fully understood |
|
| (Nanowerk Spotlight) Cobalt nanocrystals of size below 20 nm are superparamagnetic, which means they do not have a permanent magnetic moment in the absence of magnetic field, but they can respond to a magnetic field. In contrast to larger-sized cobalt particles that are ferromagnetic, superparamagnetic nanoparticles do not show hysteresis in the magnetization curves. They are being used in applications areas such as magnetic data storage and ferrofluid technology. More recently, they also find applications in biomedical research, for example labeling and separation of biological species, magnetic resonance imaging, and for guided drug delivery. | |
| Their use in large-scale commercial applications requires cobalt nanoparticles with well-defined size and shape to be prepared in large quantities. | |
| "Accurate tuning of the nanoparticle size and shape requires understanding of the mechanisms involved in particle nucleation and growth," Robin Ras, a research group leader in the Molecular Materials group at Aalto University in Finland, explains to Nanowerk. "In spite of extensive ongoing research, these mechanisms are still not fully understood owing to their complexity and interplay. Moreover, the current small-scale synthesis methods, such as the hot-injection method, can be difficult to scale to industrially relevant levels." | |
| In order to find more suitable methods for synthesizing cobalt nanoparticles, Ras and his team revisited a widely studied hot-injection synthesis of monodisperse cobalt nanoparticles and show that the particle nucleation differs from what is expected for a hot-injection synthesis. | |
| The hot-injection method is very good for producing monodisperse nanocrystals in small batches at a laboratory scale. However, when scaled to industrial level, problems arise from the difficultly to mix the precursor rapidly enough with the hot solvent to yield homogeneous nucleation and hence monodisperse nanocrystals. | |
| "We have shown that the nucleation of cobalt nanocrystals in the so-called chemical hot-injection synthesis does not take place during the precursor injection, as has been thought for past ten years" says Ras. "In an ideal hot-injection synthesis of colloidal nanocrystals, a volatile chemical precursor with a low decomposition temperature is injected into hot organic solvent containing surfactants. Nearly instantaneously, the precursor decomposes into monomers with a very high concentration (supersaturation), leading to nucleation of the nanocrystals. We showed that this ideal scheme is not valid for the well-studied hot-injection synthesis of cobalt nanocrystals." | |
| On the contrary, as the team reported in the January 26, 2011 online edition of Angewandte Chemie International Edition ("From Hot-Injection Synthesis to Heating-Up Synthesis of Cobalt Nanoparticles: Observation of Kinetically Controllable Nucleation"), they found that there is a very vigorous endothermic reaction period during which the temperature lowers significantly, few tens of seconds or a couple of minutes after the injection. | |
| "We observed that during this endothermic reaction the precursor decomposes and nanocrystals nucleate and grow into their maximum size," notes Jaakko Timonen, first author of the paper. "Importantly, we showed that there is no need to do the precursor injection at high temperature to induce rapid nucleation, but rather simple heating of the precursor with other reagents from room temperature to the nucleation temperature is enough to create nearly monodisperse nanocrystals." | |
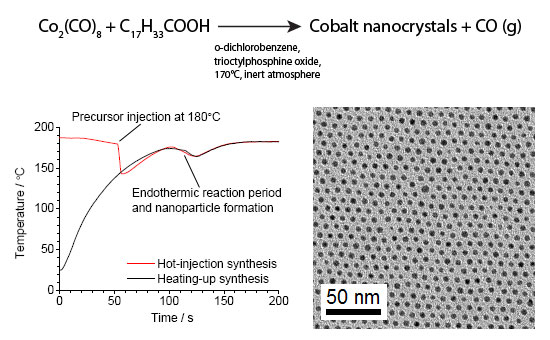 |
|
| Schematic reaction scheme. Left: Comparison between a hot-injection synthesis in which cobalt carbonyl is injected into hot (180?C) organic solvent containing surfactants (red curve) and a heating-up synthesis in which exactly same reagents are used with the exception that the cobalt carbonyl is mixed already at room temperature with the other reagents followed by heating (black curve). Around 100 seconds, both syntheses exhibit an endothermic period quite similar to each other, during which the temperature drops and the nanocrystals form. The temperature drop is very much similar in both cases and so are the nanocrystals formed, indicating that the precursor injection does not have an appreciable effect in the nanocrystal formation. Right: Cobalt nanocrystals obtained from the heating-up synthesis (black curve). (Image: Jaakko Timonen, Aalto University) | |
| It turns out that the heating-up method is much more easily scalable to industrial levels, since all reagents are mixed already at the very beginning of the reaction, followed by controlled heating and removal of the forming carbon monoxide. According to the research team, the heating-up synthesis might even be applied in a continuous flow-reactor through which the solution containing all precursors flows in a temperature gradient. | |
| As is so often the case with scientific findings, Ras and his collaborators stumbled across the problem, and its eventual solution, in an unexpected way. | |
| "In the beginning, we were trying to synthesize 12 nm cobalt nanocrystals by following the original synthesis protocol ("Colloidal Nanocrystal Shape and Size Control: The Case of Cobalt")" says Timonen. "However, when we followed the original protocol as accurately as we could, we always ended up with nanoparticles somewhere between 5-7 nm in size." | |
| Getting curious about this stubborn discrepancy, the team started to look for the reason behind it. | |
| "First, we tried varying the injection temperature, checked the procedures how we cleaned the glassware and how we handled the reagents, but they had no effect on the nanocrystal size. Eventually we started monitoring the reaction temperature and found a very peculiar reaction period a few tens of seconds or few minutes after the precursor injection, during which the reaction temperature dropped significantly and carbon monoxide evolved in the reaction vessel." | |
| Such temperature drop had not been reported in the literature earlier. The researchers took samples before and after this endothermic reaction period and found out by transmission electron microscopy analysis that the nanocrystals formed during this endothermic period. | |
| Even more interestingly, the final nanocrystal diameter could be tuned by the reaction temperature reached just before the endothermic period, which indicated that the number of nuclei formed in the hot-injection synthesis of cobalt nanoparticles depends more on temperature kinetics after the injection than on the injection itself. | |
| What is interesting is that, though the studied synthesis has been investigated extensively – over 1000 citations during the past ten years – the nucleation process apparently has not been yet completely understood. As a matter of fact, based on the Finnish team's findings, the nucleation proceeds very much differently than presumed so far. | |
| "This could be a heads-up for people working on nanocrystal synthesis, as it is possible that there are also other "hot-injection syntheses" in which the nucleation does not proceed ideally" says Timonen. "Perhaps, similar nucleation mechanisms are found in the synthesis of other types of nanoparticles. Clearly, more investigations are needed to verify this." | |
| He also points out that a more accurate description of the nanocrystal synthesis protocols reported in peer-reviewed journals would make the published results more easy to reproduce and to verify by others. | |
| "Typically, the descriptions are very brief and many aspects are not discussed, which can play even a big role after all. Nowadays, as many journals allow practically limitless supporting information to be placed on the Web to accompany the main text, there are no space-limitations to give more accurate descriptions of the recipes for nanoparticle synthesis." | |
| Researchers worldwide are putting much effort into the development of new synthesis methods of different nanocrystals ranging from oxides and semiconductors to metals and alloys. At the moment, there is a growing need to better understand the fundamental processes involved in the particle nucleation and growth. | |
| "These processes are considerably complex and the details can vary from one synthesis protocol to another," says Timonen. "We hope that our results from the cobalt nanocrystal synthesis are helpful to others as this understanding is being pursued." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
