| Posted: May 11, 2011 | |
SPIONs enable effective delivery of Malaria DNA vaccine |
|
| (Nanowerk Spotlight) Superparamagnetic iron oxide nanoparticles (SPIONs) are emerging as promising candidates for various biomedical applications such as enhanced resolution imaging or targeted drug or gene delivery due to their biocompatibility, low cost of production, ability to immobilize biological materials on their surfaces, and potential for direct targeting using external magnets. Over the past few years, researchers demonstrated that magnetofection is an appropriate tool for rapid and specific gene transfection with low dose in vitro and site-specific in vivo applications. | |
| In new work, scientists in Australia have now successfully demonstrated the use of magnetofection for the delivery of malaria DNA vaccine. Malaria is a life-threatening disease caused by a parasite called Plasmodium, which is transmitted via the bites of infected mosquitoes. In the human body, the parasites multiply in the liver, and then infect red blood cells. In 2008, there were a reported 247 million cases of malaria, which caused nearly one million deaths, mostly among African children (see WHO Fact Sheet on Malaria). | |
| The team, led by Ross Coppel at Monash University, used magnetofection to enhance the delivery of a malaria DNA vaccine. The vectors the team used were composed of SPIONs and PEI polymer complexed under different pH conditions of 4.0 and 7.0. | |
| "The procedure resulted in stable particles with a narrow size range in aqueous media, rendering them suitable for gene delivery systems," reports Coppel. "SPIONs/PEI complexes produced at acidic conditions showed the best DNA binding and gene transfection efficiency compared to those generated under neutral pH, possibly due to the protonated structure of the branched polymer that entrapped and protected the DNA. The cellular uptake of SPIONs/PEI/DNA also increased dramatically with application of external magnetic field during the gene transfection process." | |
 |
|
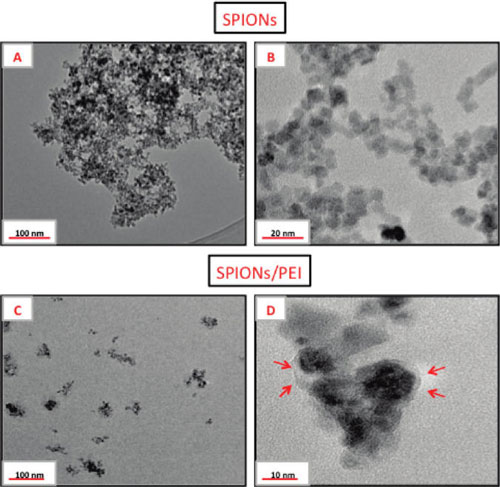
| TEM images of (A,B) as-synthesized SPIONs and (C,D) SPIONs/PEI (ratio = 10) at pH 4 displaying better dispersion (arrows indicating layer of adsorbed PEI). (Reprinted with permission from American Chemical Society) | |
| One target for growth-inhibitory antibody used in Malaria vaccine strategies is the membrane-associated 19 kDa COOH terminal fragment of merozoite surface protein (MSP119) that plays a crucial role in Plasmodium immunity, and is now a leading malaria vaccine candidate. | |
| As the team reports in a recent paper in Langmuir ("Superparamagnetic Nanoparticles for Effective Delivery of Malaria DNA Vaccine"), the high transfection potential of these nanoparticles as demonstrated by the expression of MSP119 protein in vitro indicated the possibility of using these vectors with magnetofection technique as an efficient malaria gene MSP119 delivery carrier. | |
| "The main concern for insufficient gene concentration on the target tissue for nonviral gene transfection is the insufficient accumulation of gene at the cell surface in vitro and targeting gene at the specific site in vivo," explains Coppel. "Magnetofection can be used to accumulate the magnetic gene vector on the target tissue by applying an external magnetic field." | |
| In their experiments, under the influence of magnetic field on cells, SPIONs/PEI/DNA polyplexes showed noticeably higher transfection efficiency compared to the transfection without magnetic field | |
| In vivo trials are currently underway. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
