| Posted: Aug 15, 2011 | |
Listening in on extracellular communications with a nanoplasmonic resonator |
|
| (Nanowerk Spotlight) Extracellular signaling molecules are the language that cells use to communicate with each other. These molecules transfer information not only via their chemical compositions but also through the way they are distributed in space and time throughout the cellular environment. With the development of nanosensing techniques, scientists are trying to to eavesdrop on the cellular whisper and they getting closer to deciphering extracellular signaling – an important task in understanding how cells organize themselves, for instance during organ development or immune responses. In a previous Nanowerk Spotlight we reported on a simple cell-surface sensor platform that permits signalling to be monitored within the cellular environment, in real time, in vitro and most likely also in vivo ("Sensing the niche – cells carrying sensors monitor the cellular nano-environment in real-time"). | |
| Now, researchers at UC Berkeley, UCLA, and Lawrence Berkeley National Laboratory have reported a novel sensing technique to interrogate extracellular signaling at the subcellular level. They developed a nanoplasmonic resonator array to enhance fluorescent immunoassay signals up to more than one hundred times to enable the first time submicrometer resolution quantitative mapping of endogenous cytokine secretion from an individual cell in nanoscale close to the cell. | |
| "The signal amplification of the conventional enzyme-linked immunosorbent assay (ELISA) relies on the catalyzation of the enzyme; the diffusive nature of the reporter molecules limits its applications in high-resolution sensing," Sheng Wang, a postdoc researcher in Xiang Zhang's lab at the University of California, Berkeley, explains to Nanowerk. "Conventional fluorescent immunoassay is based on spatial integration of individual signals. The signal-to-noise ratio drops drastically at the subcellular scale, the averaging of the signals over larger area scarifies the spatial resolution and hampers its ability to sense the local extracellular secretion. Therefore, the huge signal amplification through our new technique is crucial to significantly increase the signal to noise ratio for extracellular sensing down to submicron scale." | |
| Reporting their work in the July 22, 2011 online edition of Nano Letters ("Subcellular Resolution Mapping of Endogenous Cytokine Secretion by Nano-Plasmonic-Resonator Sensor Array"), first-authored by Wang, the team describes the fabrication of a tunable nanoplasmonic resonator (TNPR). This array is assembled from gold and silicon dioxide films (gold layers with 20 nm thickness sandwiching 5 nm thick silicon dioxide), placed on a grid with 500 nm distance between the 100 nm sized nanoplasmonic structures. | |
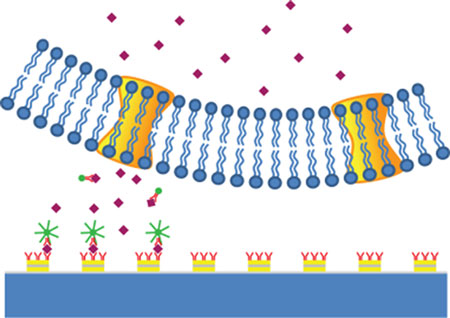 |
|
| Schematic of mapping signaling molecule secretion by a TNPR-enhanced in situ immunoassay. A TNPR array was functionalized with antibodies and had plated cell on top; a portion of the secreted signaling molecules was captured by the antibodies on TNPR. Fluorescence-conjugated antibodies were applied for detection through giant enhancement on the TNPR surface with formed antibody-antigen-antibody sandwich structure. (Image: Sheng Wang, UC Berkeley) | |
| "Our TNPRs provide unique advantages, such as extraordinary field enhancements, tunable resonance frequencies, and compatibility with a top-down mass production process (i.e. nanoimprint lithography) which enables the fabrication of large numbers of uniform nanoplasmonic structures with controllable resonance spectra and electromagnetic field enhancements," says Wang. | |
| The TNPR is a plasmonic resonator structure that enhances the local optical field, i.e. the local light intensity – 'local' meaning a layer of 10 nm from the gold surface. The team says that TNPR enhancement of in situ immunoassays suggests a possible method to significantly enhance a fluorescent detection signal along with markedly improving the signal-to-noise ratio for accurate biosensing. | |
| To showcase the capabilities of such a TNPR-enhanced in situ immunoassay, Wang and his collaborators demonstrated subcellular resolution mapping of cytokine secretion. | |
| "There is a hypothesis that during interleukin-2 (IL-2 is a type of cytokine immune system signaling molecule) autocrine processes (regulating a cell through self secreted factors feeding back on its own), the local concentration of IL-2 is much higher than the global concentration – but for many years there was only theoretical analysis and indirect evidence available due to the lack of a method to detect local IL-2 concentration" explains Wang. "Our work utilizing the new technique finally validates this hypothesis through direct measurement. We show that the local IL-2 concentration near the cell can be 100,000 to 1,000,000 higher than the global concentration in the media." | |
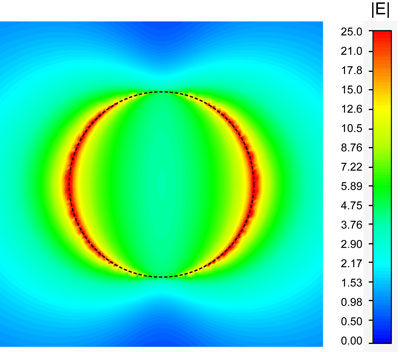 |
|
| Spatial distribution of the optical intensity enhancement at the plane above the top surface of a TNPR (100 nm diameter). (Image: Sheng Wang, UC Berkeley) | |
| The researchers note that an important future application for the TNPR technique is to determine the signaling molecule spatial distribution between polarized cells, such as those during the signaling and communication events between neuronal or immunological synapses. | |
| "It will also enable the simultaneous detection, spatial mapping, and quantification of multiple, distinct signaling molecules when TNPRs are engineered with multiple resonance peaks for multiple color enhancements" Wang points out. "Our technique opens a new avenue in sensing and quantifying subcellular level signaling molecule secretion and could have broad impact for deciphering extracellular signaling and function." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
