| Posted: Aug 18, 2011 | |
Nanofluidic device allows protein detection with unprecedented sensitivity |
|
| (Nanowerk Spotlight) Molecular separations are extremely important in a wide range of technologies, from conventional proteomics to pathogen detection and DNA fingerprinting. A complication arises from the fact that molecular components in mixtures can span an enormous range of concentration. Conventional approaches such as antibody depletion are not sensitive enough to detect numerous medically significant biomarkers, whose incidence in blood could be as much as a trillion times less abundant than the most plentiful protein, albumin. | |
| "This limitation prevents the isolation, characterization, and discovery of millions of new proteins where key disease markers could be identified," David Inglis, a Research Associate in the Department of Physics at Macquarie University, tells Nanowerk. "Overcoming this barrier requires new approaches to analytical detection that minimize sample preprocessing steps while achieving high throughput with very high levels of sensitivity." | |
| A recent paper in Angewandte Chemie International Edition ("Simultaneous Concentration and Separation of Proteins in a Nanochannel"), first authored by Inglis, shows a new path in miniaturized molecular separations. It describes a new device that demonstrates simultaneous concentration and separation of proteins by conductivity gradient focusing. Concentration and separation take place in an electric-field-driven 120 nm deep nanochannel that supports a stable salt and conductivity gradient. The results show that relevant proteins can be concentrated to detectable levels. | |
| This work demonstrates that a simple nanochannel that connects to chemically different solutions creates a stable and robust gradient between those two solutions. This gradient can then be used to perform separations and selective concentration enhancement. Such processes have previously been demonstrated in systems that create the gradient in more complex ways. | |
| "Our work is based on the idea that, by placing the gradient and the molecules in the nanochannel, the gradient is extended, thus allowing separation of molecules while they are being concentrated" explains Inglis. "Furthermore, rather than relying on concentration polarization, we use a nanochannel that connects a high-conductivity and a low-conductivity microchannel to impose an electric field gradient." | |
 |
|
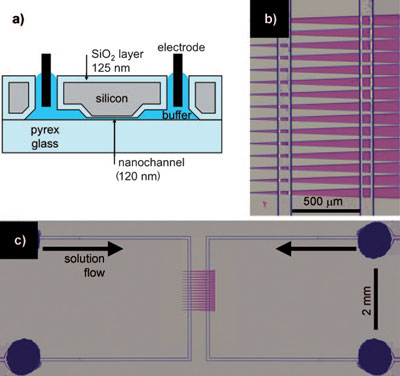
| Device description. a) Cross-section of the device showing a 120 nm-deep nanochannel. b) Top view of the nanochannels (purple) connecting two microchannels. The four vertical lines are microchannel edges. c) Top view of the entire device. The four dark circles (one is obscured) are holes cut through the silicon for liquid and electrode access. (Reprinted with permission from Wiley-VCH Verlag) | |
| As illustrated in the figure above, the device consists of a pair of microchannels (8.6 µm deep) connected by 25 parallel nanochannels (each 120 nm deep). The nanochannels are almost three times as wide at the right end (62 mm) than at the left (22 nm), with a 2° half-angle. | |
| As Inglis explains, low- and high-salt buffers are continuously transported through the left and right microchannel, respectively. "The proteins under investigation are added to the right-hand, high-salt microchannel. Capillary forces and evaporation at the downstream end continuously drive the fluid in the microchannels at approximately 5 nL per minute. This flow sustains the salt concentration gradient despite the buffer exchange through the nanochannels. A positive voltage is applied to electrodes in the right-hand, high-salt microchannel, while the left-hand, low-salt channel is grounded. Electro-osmotic flow carries the buffer with dissolved proteins into the nanochannel, where they may be trapped." | |
| Since the bulk protein concentration is much less than the salt concentration, the presence of the protein does not affect the gradient. | |
 |
|
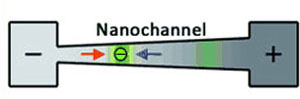
| The fundamental concepts of conductivity gradient focusing. A conductivity gradient is established in a nanochannel connecting two reservoirs. At the high-concentration salt (+) side, the electro-osmotic force dominates (blue arrow), while at the low-salt (-) side the electrophoretic force dominates (red arrow). Proteins (green) are trapped in positions within the nanochannel wherever the net force is zero. (Reprinted with permission from Wiley-VCH Verlag) | |
| The researchers demonstrated their device by simultaneously concentrating and separating two fluorescent proteins (R-phycoerythrin and Dylight488-labelled streptavidin). Initially, the two proteins are mixed high-salt microchannel. After applying a voltage of 4V for 110 seconds, both proteins are well-resolved (see image below). | |
 |
|
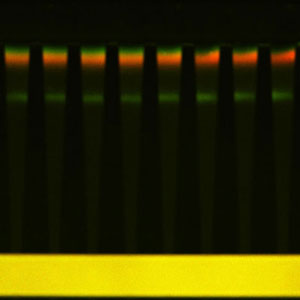
| Image showing simultaneous separation and concentration of a green and red protein, and DNA (fluorescent green close to the middle of the channel). DNA is much more negatively charged than the proteins so traps much closer to the high-salt, positive-voltage microchannel. (Image: Dr. David Inglis, Macquarie University) | |
| Inglis says that the R-phycoerythrin molecules have been concentrated by a factor of 60 and the Dyl-Strep molecules by a factor of 51. " We calculated concentration enhancement as the peak intensity divided by the microchannel intensity times a geometric factor that corrects the fluorescence intensity for the depths of the microchannels and nanochannels." | |
| Applications of this novel device will most likely be found in proteomics, especially in the integration of separation and electro spray mass spectrometry; mobility shift immunoassays for molecular detection; and detection of nucleic acids using fluorescent molecular beacons. | |
| Taking their work forward, Inglis and his collaborators are now trying to develop better control over the range of molecules that are trapped and they plan to investigate new materials and coatings with low protein adsorption. | |
| Below is a brief time-lapse movie showing the flow of proteins into the nanochannel where they are concentrated and separated into two clearly resolved bands near the low-salt end. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
