| Posted: Aug 26, 2011 | |
Measuring the force of a single synthetic small molecule |
|
| (Nanowerk Spotlight) Previously, synthetic molecular machines have been used to perform mechanical tasks collectively, such as move liquid droplets uphill against the force of gravity ("Macroscopic transport by synthetic molecular machines"), rotate microscale objects using liquid crystals doped with synthetic motor-molecules ("Molecular machines: Nanomotor rotates microscale objects") and bend cantilevers ("Linear Artificial Molecular Muscles"). However, all these tasks are achieved by the collective action of billions and billions of molecular machines. Observing the mechanical behavior of an individual molecule is much more difficult. | |
| In biology, however, molecular machines – for example the kinesin and dyenin motor proteins – often function individually (each kinesin molecule carries its cargo – typically a vesicle filled with newly made proteins – from the nucleus to the periphery of the cell). In the last 15 years, major advances in instrumentation such as optical tweezers and force microscopies have allowed motor proteins and many other single biomolecules to be observed individually, including their dynamics and how they behave mechanically. Amazing insights have been gained from these experiments in terms of understanding the mechanisms of how motor proteins move and perform tasks. | |
| "Synthetic molecular machines, however, are often ten times smaller in each dimension (1000x smaller in molecular weight) than motor proteins and previously no one has managed to use single molecule techniques to look at how the components move in synthetic molecular machines," David Leigh, Forbes Professor of Organic Chemistry at The University of Edinburgh, explains to Nanowerk. "By using very sensitive atomic force microscopy (AFM) experiments, Anne-Sophie Duwez (a professor in NanoChemistry and Molecular Systems at the University of Liège) and our group were able to address the movement of the ring in individual rotaxane molecules." | |
| Reporting their findings in the August 21, 2011 online edition of Nature Nanotechnology ("A single synthetic small molecule that generates force against a load"), Leigh's and Duwez's team show that a single synthetic small molecule can generate force against a load of 30 piconewton (pN). | |
| Rotaxanes are molecules consisting of a ring threaded onto a linear axle. The ring can, in principle, be moved between different sites on the axle and this movement in some cases used to generate mechanical force (i.e. a synthetic molecular machine). | |
 |
|
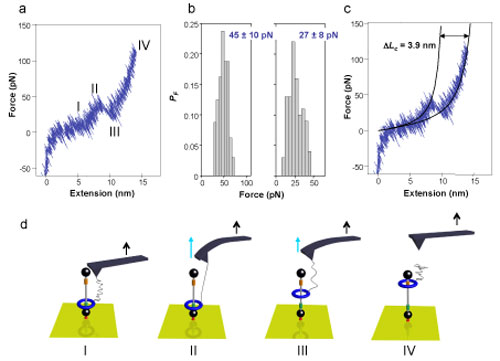
| Experimental AFM pulling curve of the rotaxane-PEO molecule and schematic representation of the experiment. a, High resolution force-extension curve in TCE. b, Histograms of the rupture forces for the hydrogen bonds in TCE (average ± s.d., n=318) (left) and in DMF (average ± s.d., n=249) (right) at a loading rate of 500 pN s-1. c, Force-extension curve with worm-like chain model fits to the data (thin solid lines) with an increase in length (ΔLc) of the molecule after the rupture of the hydrogen bonds of 3.9 nm. d, Interpretation of the sequence of the events taking place when pulling on the rotaxane-PEO. The black arrows show the direction of the cantilever displacement. The blue arrows show the direction of the force exerted on the ring. (I): progressive stretching of the PEO tether. (II): once the force exerted on the tether exceeds the force of the hydrogen bonds between the ring and the fumaramide site, the hydrogen bonds break. (III): after the rupture, the ring is free to move along the thread, the tension in the PEO backbone is partly released and the force decreases (III) until the displacement of the cantilever increases again the tension in the PEO tether. (IV): further cantilever displacement continues the stretching of PEO until the force exceeds the interaction strength of the chain with the tip, which leads to detachment. (Image: Leigh Group, The University of Edinburgh) | |
| "We built a rotaxane with a relatively long axle (4.5 nm) and a binding site at one end of the axle where the ring prefers to reside as it can form up to four hydrogen bonds there," Leigh describes the experiment. "We attached that end of the rotaxane axle to a gold surface. To the ring of the rotaxane we attached a polymer chain which was connected at the other end to the AFM tip. As the AFM tip is pulled away, the polymer chain straightens out and the force measured by the AFM experiment at that stage was typical of similar AFM experiments on polymer unfolding." | |
| "However, once the polymer chain is unfolded, if we continued to pull with the AFM tip then the ring of the rotaxane is pulled away from the hydrogen bonding site and is pulled up the axle," he continues. "Amazingly, this process can be followed on a single molecule by seeing how the force resisting the tip changes. Even more remarkably, once the ring is pulled to the top of the axle of the rotaxane, if we stop pulling and allow the ring to move by random thermal motion within the flexibility of movement offered by the polymer chain, eventually it finds its original hydrogen bonding site at the bottom of the axle and pulls on the polymer chain and tip to allow it to stay at that site, a single rotaxane molecule exerting a mechanical force on an AFM tip." | |
| By doing the pull-relax cycles hundreds of times, the researchers could build up a statistical analysis of the force generated by the synthetic molecular machines. They found that the ring of a single rotaxane molecule can move to its preferred binding site against a resisting force of 30 pN. | |
| In other words, a single rotaxane molecule was measured to pull on the AFM tip with a force of 30 pN. That's not dissimilar to the forces measured for single biological machines such as myosin, kinesin, ATPases and DNA- and RNA-polymerases which tend to be in the range 5-60 pN. | |
| This is the first time that the force generated by an individual synthetic molecular machine has been directly measured. | |
| In previous work, single-molecule measurement techniques such as optical tweezers or force clamp AFM have provided extraordinary insights into the dynamic behavior of motor proteins and other biomolecules that have revolutionized scientists' understanding of their mechanisms. Now the same techniques can be applied to synthetic small-molecule systems. | |
| Now, Leigh and his team plan to examine the behavior of other small-molecule synthetic molecular machines like synthetic molecular walkers in the same way. They also plan to explore the use of other single molecule techniques (e.g. optical tweezers) that have been so successful in probing biological systems for observing and measuring the dynamic behavior of synthetic small-molecule machines. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
