| Posted: Nov 21, 2011 | |
A new tool to track the intracellular trafficking of nanomedicines |
|
| (Nanowerk Spotlight) In nanomedicine, nanoparticles are used as vehicles for efficiently delivering therapeutic nucleic acids, such as disease-fighting genes and small interfering RNA (siRNA) molecules, into cells. But getting nanomedicines to their target sites inside cells is not the only challenge. It also is necessary to assess the intracellular processing of nanomedicines and the efficacy of their payload delivery – a task that is not exactly trivial given the complexity and dynamics of the mechanisms of endocytosis (the process of molecules being absorbed into cells) and intracellular trafficking. | |
| Researchers are therefore trying to develop robust and reliable tools to characterize and evaluate the intracellular processing of administered nanomedicines. As part of this effort, scientists have now introduced a quantitative approach to study live-cell endosomal colocalization dynamics of nanomedicines for gene delivery, based on single-particle tracking (SPT) and trajectory-correlation. | |
| Reporting their work in a recent issue of ACS Nano ("Dynamic Colocalization Microscopy To Characterize Intracellular Trafficking of Nanomedicines"), they conclude that dynamic colocalization is a promising tool to map and understand intracellular endosomal trafficking of nanomedicines. | |
| "In fluorescence microscopy, colocalization or overlap of different labels/markers is a common investigation in order to draw conclusions on interactions or association of the labelled structures," Dries Vercauteren, a post-doctoral researcher in the Research Group on Nanomedicines at Ghent University in Belgium, explains to Nanowerk. "Often, this is done in fixed cells – with the help of immunostaining – which can cause artefacts because of the chemical procedures. Colocalization is often evaluated qualitatively and often on static images, which can indicate false colocalization because of incidental overlap of the fluorescent signals (because of limited resolution in light microscopy). To avoid all of these issues, fluorescence colocalization can be studied in living cells, and in case of motile structures, e.g. endosomes, be identified through correlated movement." | |
 |
|
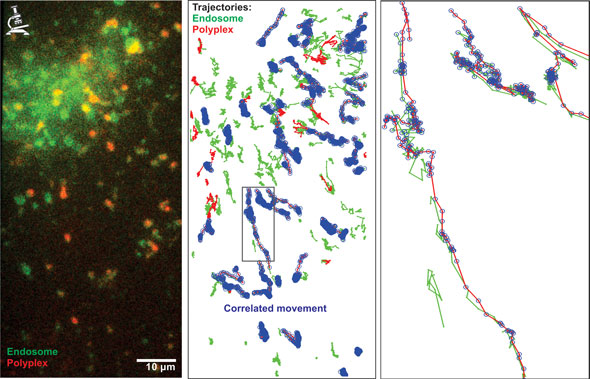
| The dynamic colocalization algorithm detects correlated movement of fluorescently labelled objects. EGFP-LAMP1 (in green)-expressing retinal cells were incubated with nanomedicines for retinal gene therapy, carrying fluorescently labelled nucleic acids (in red). These nanomedicines are internalized via endocytosis and movies of these cells were acquired in realtime (left). With the help of tracking software, both green and red objects were tracked throughout the movie and the tracks of the green and red channel were evaluated for correlated movement (right, correlated tracks are indicated with blue circles). The percentage of red tracks that correlate with green tracks finally results in the dynamic colocalization value and indicated the presence of nanomedicines in LAMP1-positive endosomes at that given time point. (Image: Dr. Dries Vercauteren, Ghent University) | |
| For their work, the team, led by Kevin Braeckmans, used quantitative live-cell fluorescence colocalization microscopy to study the intracellular trafficking of polymeric gene complexes (polyplexes) in living retinal pigment epithelium (RPE) cells (which are attractive targets for ocular gene therapy). | |
| "We were able to confirm the accumulation of endocytosed non-viral nanoparticles/nanomedicines for nucleic acid delivery in lysosomes, which restricts further intended release of the active compounds in the cellular interior," says Vercauteren. "Furthermore, we developed a method that was able to map quantitatively the kinetics of this intracellular, endosomal trafficking in living cells. Finally, we provided first evidence of the involvement of autophagy in this process of endolysosomal accumulation." | |
| The researchers' efforts were inspired by findings that, despite the ability to overload the endosomal system of all cells with these nanomedicines, there was no clear correlation with the biological effect. Despite the intracellular presence of nanomedicines in high quantities, 24 hours after initial addition of the nanomedicines, no additional increase in biological effect could be observed, indicating significant loss of functionality of the internalized fraction of nanomedicines. | |
| Apart from the implementation of a new method to study the process of endosomal trafficking of nanoparticles in living cells, another innovative aspect of this work was to obtain the time information of these nanoparticles as they are trafficked to the lysosomes and the identification of the specific endosomes that are involved in this endosomal trafficking. | |
| Moreover, as Vercauteren points out, "our work has proven that endosomal escape/cytosolic release is a critical parameter in cellular nucleic acid delivery, as microinjection of these nanomedicines in the cytosol of the cells led to the desired gene expression within one hour, regardless of cell division. Finally, the indication that autophagy may be key regulating process and defense mechanism against these foreign invaders – the nanomedicines – might provide new insights in the efficacy of non-viral nucleic acid transfer." | |
| Example of dual-color live-cell acquisition of a human RPE cell, expressing EGFP-flotillin-2 (green), 4.5 hours after exposure to DNA-polyplexes (red). The movie takes 10 minutes and is acquired at a frame rate of 2 fps, which is speeded up here fivefold to 10 fps. The field of view is 40 by 19.5 µm. (Video: Dr. Dries Vercauteren, Ghent University) | |
| One interesting application of this method, that allows quantification of endosomal localization in the time domain and lysosomal accumulation in living cells, is that it would enable the comparison of different formulations of nanomedicines at their level of endosomal trafficking and lysosomal accumulation. | |
| Equally important, the same method could be applied to find answers to the observations why certain nanocarrier formulations work well in one cell type, and less in other cell types, aiding the development of more effective nanomedicines. | |
| The team notes that the characterization of endosomal trafficking of endocytosed nanomedicines in a spatio-temporal way could be improved by introducing confocality, improving signal-to-noise ratios in the images and therefore allow the detection of objects with lower intensities, objects that are highly concentrated, like in the perinuclear region and objects that are close to the plasma membrane. | |
| "Colocalization studies with more than one endosomal marker and nanomedicines in a simultaneous way would allow detection of fusion of various types of endosomes that contain nanomedicines and would allow quantification of overlap of the various endosomal markers," Vercauteren describes on aspect of future research. "Besides the technical challenges, this could imply the evaluation of various nanocarriers for the kinetics of their lysosomal accumulation, in order to identify the nanocarriers that slow down or avoid unwanted lysosomal accumulation." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
