| Posted: Dec 06, 2011 | |
Carbon nanotube-coated sponge makes an excellent supercapacitor |
|
| (Nanowerk Spotlight) Ultra- or supercapacitors are emerging as a key enabling storage technology for use in fuel-efficient transport as well as in renewable energy (for instance as power grid buffer). These devices combine the advantages of conventional capacitors – they can rapidly deliver high current densities on demand – and batteries – they can store a large amount of electrical energy. | |
| Supercapacitors offer a low-cost alternative source of energy to replace rechargeable batteries for various applications, such as power tools, mobile electronics, and electric vehicles. A number of auto makers are exploring the concept of combining supercapacitors with Li-ion batteries as a next generation energy storage system for their electric hybrid vehicles. Although the energy density of capacitors is quite low compared to batteries, their power density is much higher, allowing them to provide bursts of electric energy that can help the new generation of cars to accelerate at comparable or better rates than traditional petrol-only engine vehicles, while achieving a significantly reduced fuel consumption (read more about supercapacitors and other nanotechnologies to mitigate global warming). | |
| Researchers have now fabricated novel high-performance sponge supercapacitors using a simple and scalable method. Reporting their work in a recent edition of Nano Letters ("High-Performance Nanostructured Supercapacitors on a Sponge"), a team at King Abdullah University of Science and Technology (KAUST) in Saudi Arabia, led by Husam N. Alshareef, shows that three-dimensional electrodes potentially have a huge advantage over conventional mixed electrode materials. | |
| "We showed supercapacitor electrodes fabricated on a macroporous structure give a boost to the speed and specific capacitance of the supercapacitors," Alshareef tells Nanowerk. "We worked with sponges because they offer novel exciting characteristics different from paper and textile: first, sponge has much more uniform size of macropores. Second, the cellulose or polyester fibers are interconnected virtually free of junctions. Therefore, continuous coating of conducting nanomaterials is much easier since there are no junctions to cross." | |
 |
|
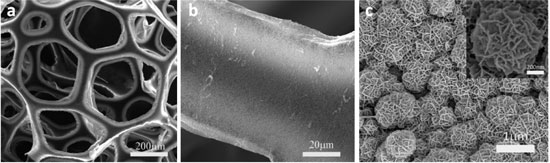
| Characterizations of MnO2-CNT-sponge electrodes: (a) an overall view of 3D macroporous hierarchical MnO2-CNT-sponge electrode; (b) MnO2 uniformly deposited on the skeleton of CNT-sponge; (c) high magnification of porous MnO2 nanoparticles on CNT-sponge, inset shows morphology of an individual MnO2 flower-like particle. (Reprinted with permission from American Chemical Society) | |
| The sponge-based supercapacitors were designed and fabricated by the KAUST team using a simple and scalable method. Their fabrication process consisted of four simple steps: First, a piece of commercially available sponge was cleaned by water and acetone; then, after drying, it was cut into small ribbons; these ribbons were coated with a carbon nanotube (CNT) ink; and finally, the researchers electrodeposited manganese dioxide (MnO2) nanoparticles onto the CNT-coated sponge. | |
| "Due to the mechanical flexibility of carbon nanotubes and strong van der Waals interactions between the macroporous sponge cellulose and carbon nanotubes, the nanotubes can be easily coated onto the skeleton of a sponge, rendering the insulating sponge highly conductive by a simple dipping and drying process" explains Alshareef. | |
| Examining the CNT-coated sponge, the researchers found that it still maintained a hierarchical macroporous nature where its intricate assembly of pores remained open to allow the flow of electrolyte. The carbon nanotubes formed a thin layer wrapped around the skeleton of the sponge. | |
| "We also tested the mechanical resilience of the CNT-sponge skeleton by folding, twisting, and stretching it repeatedly," says Alshareef. "After all the mechanical tests, the sponge always reverted to its original shape without any permanent deformation." | |
| The team also tested the electrochemical performance of their sponge supercapacitors. "The macroporous nature of the sponge along with the porous nature of the electrodeposited MnO2 nanoparticles provided a double porous electrode structure giving good conductivity and full accessibility of electrolyte to MnO2, improving the performances of MnO2-CNT-sponge supercapacitors dramatically," says Alshareef. "We found that our sponge supercapacitor exhibits high specific capacitance, ultrafast charge-discharge rate, excellent cycling stability, and good energy and power density, making it a promising electrode for future energy storage systems." | |
| In what could be an interesting aspect for small mobile electronic appliances, the finished supercapacitor sponge turns out to be much lighter than a rigid metal or other flexible substrates with the same area: a sponge with an area of 2 square centimeters and a thickness of 1 millimeter weighs only about 10 mg. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
