| Posted: Dec 08, 2011 | |
Self-propelled microrockets detect dangerous bacteria in food, clinical and environmental samples |
|
| (Nanowerk Spotlight) No matter if you are into big, fat hamburgers or eat entirely vegetarian, nibbling on spinach leaves and celery stalks, some food borne pathogens will sooner or later get you. The Centers for Disease Control and Prevention (CDC) estimates that in the United States alone, food borne pathogens cause approximately 76 million illnesses, 325,000 hospitalizations, and 5,000 deaths. If that is not scary enough for you, take a look at the U.S. Food and Drug Administration's Bad Bug Book that lists food borne pathogenic microorganisms and natural toxins. | |
| Early detection of food borne pathogenic bacteria is critical to prevent disease outbreaks and preserve public health. This has led to urgent demands to develop highly efficient strategies for isolating and detecting this microorganism in connection to food safety, medical diagnostics, water quality, and counter-terrorism. | |
| E. coli and other pathogenic bacteria are commonly detected using traditional culture techniques, microscopy, luminescence, enzyme-linked immunosorbent assay (ELISA), biochemical tests and/or the polymerase chain reaction (PCR). These techniques, however, are time-consuming, labor-intensive, and inadequate as they lack the ability to detect bacteria in real time. Thus, there is an urgent need for alternative platforms for the rapid, sensitive, reliable and simple isolation and detection of E. coli and other pathogens. | |
| Taking a novel approach to isolating pathogenic bacteria from complex clinical, environmental and food samples, researchers have developed a nanomotor strategy that involves the movement of lectin-functionalized microengines. Receptor-functionalized nanoswimmers offer direct and rapid target isolation from raw biological samples without preparatory and washing steps. | |
| This work has been published on December 5, 2011 as "Just Accepted Manuscript" in Nano Letters ("Bacterial Isolation by Lectin-Modified Microengines"). | |
| "We have demonstrated the use of new synthetic template-prepared microrockets ("Highly Efficient Catalytic Microengines: Template Electrosynthesis of Polyaniline/Platinum Microtubes"), functionalized with lectin receptors, for the efficient isolation of target bacteria from diverse real samples," Joseph Wang, a professor in the Department of Nanoengineering at University California San Diego (UCSD), tells Nanowerk. "These modified self-propelled microengines offer very attractive capabilities for autonomous loading, directional transport and bacterial unloading – 'catch, transport and release' – towards subsequent re-use, along with efficient and simultaneous transport of drug nanocarriers. The new smaller microengines allows convenient label-free real-time visualization of the binding event and differentiation against non-target cells without the need for additional tagging." | |
 |
|
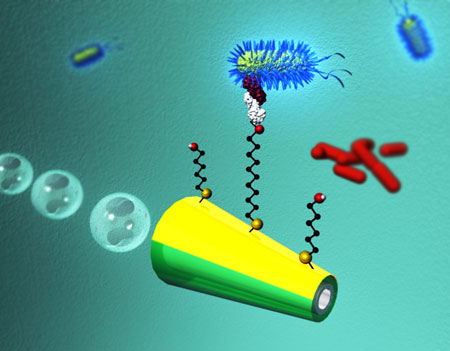
| Micromachine-based isolation of bacterial targets from complex samples involving capture and release abilities of lectin-modified microengines. (Image: Wang Group, Department of Nanoengineering, University California San Diego) | |
| The motion and power of self-propelled synthetic and natural nano/microscale motors have been exploited recently by the UCSD team as an attractive route for transporting target biomaterials, such as cancer cells or nucleic acids ("Motion-driven sensing and biosensing using electrochemically propelled nanomotors"), but not for the capture and transport of pathogenic bacteria. | |
| Wang's team also demonstrated, for the first time, the ability to capture and transport simultaneously the target bacteria along with drug-carrier polymeric spheres (towards a theranostics operation), as well as a chemically-triggered unloading (release) operation. | |
| The efficient bacterial isolation platform that the team developed relies on the attractive behavior of their microrockets along with their functionalization with lectin receptors. | |
| "Lectins are readily available sugar-binding proteins that offer an attractive route for recognizing carbohydrate constituents of bacterial surface, via selective binding to cell-wall mono- and oligosaccharide components," explains Wang. "For example, ConA, the lectin extracted from Canavalia ensiformis that we used in this work, is a mannose- and glucose-binding protein that is capable of recognizing specific terminal carbohydrates of Gram-negative bacteria such as the E. coli surface polysaccharides." | |
| Although lectins have been recently used as the biosensor recognition elements for bacterial detection, their use in connection to nanomachines or nanoscale motion-based isolation is a novel approach. | |
| As illustrated in the figure above, the team's nanoscale bacteria isolation strategy utilizes the movement of ConA-functionalized microengines to scour, interact and isolate pathogenic bacteria from distinct complex samples. After bacteria have been captured, they then can be released in a controlled fashion by moving the microrocket through a low-pH glycine solution (which dissociates the lectin-bacteria complex). | |
| Finally, but equally important, the microrockets are dual-action, i.e. besides capturing and transporting target bacteria they can simultaneously transport polymeric drug-carrier spheres to provide 'on-the-spot' therapeutic action. | |
| "The diverse capabilities of our lectin-modified microrockets make them extremely attractive for a wide-range of fields, including food and water safety, infectious disease diagnostics, biodefense, and clinical therapy treatments," says Wang. "The incorporation of such a microengine-based bacterial isolation protocol into microchannel networks could lead to microchip operations involving real-time isolation of specific bacteria, its lysis and unequivocally identification (by 16S rRNA gene analysis)." | |
| Overall, the new microengine 'catch-transport-release' platform presents a unique approach for meeting the need for rapid, direct and real time isolation of biological agents. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
