| Posted: Dec 23, 2011 | |
A step toward a better understanding of how copper catalysts interact with carbon nanomaterials |
|
| (Nanowerk Spotlight) Carbon nanomaterials with different morphologies and internal structures – including carbon nanotubes, carbon nanofibers, and graphene – have been synthesized by catalytic decomposition of hydrocarbon gases. A number of parameters are known to affect the synthesis of carbon nanomaterials, such as the composition and size of the catalysts, type of hydrocarbon gas, temperature, and reaction time. Different carbon nanomaterials having various carbon atomic configurations demonstrate different physical and chemical properties. As a result, it is critical to synthesize carbon nanomaterials with controlled morphology and internal structures for their potential applications as building blocks for nanoscale electronics and photonics, catalyst supports for fuel cells, non-viral carriers for delivering biomolecules into cells, biomedical imaging, and additives for reinforced composite materials. | |
| Notwithstanding all the advances that researchers have made in fabricating carbon nanomaterials, it remains challenging to generate a graphene sheet with a defined number of layers or a batch of carbon nanotubes with the same chirality and diameter. | |
| "In order to overcome these barriers, we need to investigate the interactions between catalysts and carbon nanomaterials to understand how the catalyst facilitate the growth of carbon nanomaterials and, thereby, obtain carbon nanomaterials with controlled properties through tailoring of their catalyst parameters," Lifeng Dong, a Taishan Scholar Overseas Distinguished Professor at Qingdao University of Science and Technology and an Associate Professor in the Department of Physics, Astronomy, and Materials Science at Missouri State University, explains to Nanowerk. | |
| In a previous Nanowerk Spotlight ("The role of surfactants in carbon nanotube toxicity") we reported on an earlier study by Dong and his team that showed that purified carbon nanotubes did not affect 1321N1 human astrocytoma cells adversely. Sometimes, the cytotoxicity effects of carbon nanotubes result from catalyst particles within the nanotubes (see also: "Comparing apples with oranges – the problem of nanotubes risk assessment"). | |
| Dong points out that it is critical to understand interactions between catalyst particles and carbon nanomaterials for the synthesis of carbon nanomaterials with controlled morphology and internal structures for their potential applications in nanoscale electronics, optoelectronics, catalyst supports for direct ethanol/methanol/hydrogen fuel cells, and medicines. Also, it is important to eliminate catalyst particles from carbon nanomaterials for certain applications, such as carbon nanomaterials as transporters for biomolecules into cells. | |
| In new work, Dong and his team, together with researchers from the National Center for Electron Microscopy at Lawrence Berkeley National Laboratory, demonstrate that, due to the low solubility of copper (Cu) in carbon and its affinity with oxygen (O), single-crystal copper catalysts dissociate into small cuprous oxide (Cu2O) nanoparticles after the growth of carbon nanofibers, and Cu2O nanoparticles ultimately localize on the fiber surfaces. | |
 |
|
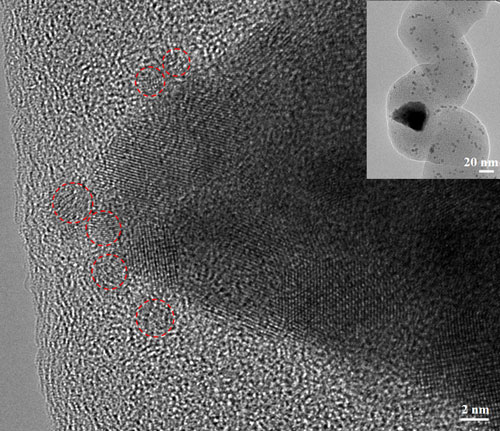
| High resolution transmission electron microscopy image shows that a central copper catalyst particle possesses a faceted shape (top right inset), but has dissociated into small "satellite" nanoparticles. These small particles have diameters ranging from 2 to 6 nm and exhibit different crystal orientations relative to each other. A few small particles with a diameter of less than 2 nm (labeled with dashed-line circles) are observed within the amorphous carbon fiber. (Image: Dr. Lifeng Dong, Qingdao University of Science and Technology and Missouri State University) | |
| For this work the scientists used one of the few globally available, double-aberration-corrected (scanning) transmission electron microscope (STEM/TEM) at an acceleration voltage of only 80 kV. | |
| This new finding, reported in the December 16, 2011 online edition of Nanotechnology ("Direct imaging of copper catalyst migration inside helical carbon nanofibers"; free article), is a step toward a better understanding of the interactions between copper catalysts and carbon nanomaterials and could suggest a simple and effective method for eliminating copper impurities from the fibers. | |
| "Graphene, carbon nanotubes, and carbon nanofibers have been synthesized successfully by the catalytic decomposition of various hydrocarbon gases with the use of copper catalysts under varying reaction conditions, but no report exist, to our knowledge, concerning interactions between copper catalysts and carbon nanofibers after the initial fiber formation," says Dong. "Accordingly, we report here for the first time an investigation of post-fiber formation interactions between copper catalysts and carbon nanofibers, using high resolution TEM/STEM, electron energy-loss spectroscopy (EELS), and energy-filtered TEM (EFTEM). We find that copper catalysts disassociate into smaller oxide clusters, which suggests future strategies for nanofiber purification and contributes to a better understanding of the interactions between copper catalysts and carbon nanomaterials." | |
| In order to fully understand the interactions between catalyst particles and carbon nanomaterials, the researchers will investigate the dynamics and kinetics of the formation of satellite Cu2O particles insides carbon nanofibers at different temperatures as well as the interactions between Cu catalyst and graphene. | |
| "Due to their small sizes and sensitivity to electron beam irradiation, it is very challenging to image carbon nanofibers and graphene sheets," says Dong. "Even with the use of state-of-the-art double-aberration-corrected TEAM I (scanning) transmission electron microscope at 80 kV acceleration potential, carbon nanofibers were not stable under the focused electron beam using scanning transmission electron microscopy (STEM) mode, and the crystal structure of copper catalyst needs to be characterized using high resolution transmission electron microscopy (TEM) mode." | |
| Background to this work | |
| In October, 2007, Dr. Dong was awarded the Visiting Scientist Fellowship from the National Center for Electron Microscopy (NCEM) located at the Lawrence Berkeley National Laboratory. Consequently, he has had opportunities to work with leading scientists at the NCEM including Dr. Peter Ercius, Chengyu Song, Dr. Uli Dahmen, and Dr. Thomas Duden and uses state-of-the-art TEAM I electron microscopy facility to investigate the interactions between copper catalysts and carbon nanofibers. | |
| In October, 2009, Dr. Dong was awarded the Taishan Scholar Overseas Distinguished Professor of the College of Materials Science and Engineering at Qingdao University of Science and Technology (QUST) from the Shandong Province Government, P.R. China and thereby has opportunities to work with Professor Zuolin Cui, Dr. Liyan Yu, and Hongzhou Dong at QUST. Carbon nanofibers were synthesized at the QUST and were characterized at the NCEM. The findings resulted from a collaborative research project among QUST, NCEM and Missouri State University. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
