| Posted: Aug 21, 2007 | |
Wet nanotechnology - living transistors with nanofluidic diodes |
|
| (Nanowerk Spotlight) Ion channels are proteins with a hole down their middle that are the gatekeepers for cells. Ion channels control an enormous range of biological function in health and disease. In channels with a diameter greater than 100 nm, the interaction between the channel wall and electrolyte solution hardly affects the flow of ions. When the channel diameter enters the the <10 nm range, things change dramatically, however. Then, the interaction between the solution and channel wall starts to dominate ionic flow and ion transport through such narrow, nano-scaled channels is dominated by electrostatics. | |
| The same is true for biological ion channels where charged amino residues in the selectivity filter determine the ionic flow through the channel, along with the dielectric charge on the channel wall, and the concentrations and potential in the bulk solution. The role electrostatics play in biological pores has been confirmed by numerous mutation studies where amino acids residues in the selectivity filter were replaced by others. Ion channels have simple enough structure that they can be analyzed with the usual tools of physical science. | |
| With that analysis in hand, researchers are trying to design practical machines that use ion channels. By exploiting the electrostatics in nanochannels a group of US and Dutch scientists managed to make a diode. Like a solid-state diode allows current flow in one direction, the ionic equivalent they designed can be used to direct the flow of ions across a membrane that separates two electrolyte solutions. | |
| Now that they know how to manipulate the ion selectivity in these devices, they hope to be able one day to selectively amplify currents carried by individual chemical species - a stunning prospect for molecular nanoelectronics. | |
| Prof. Bob Eisenberg at Rush University Medical Center in Chicago has been one of the frontrunners in studying the way the potential profile in an ion channel directs the ion flow through the channel. This potential arises not only from the permanent charge present in the selectivity filter of the channel but also from the mobile ion species that, while passing through, reside in the channel. This is one of the key features of the Poisson-Nernst-Planck (PNP) approach developed by Bob Eisenberg that states that the equations that govern the potential (Poisson) and ionic flux (Nernst-Planck in the ideal case) has to be solved simultaneously using an iterative calculation. | |
| "Our work may challenges us to design and build biodevices with even more sophisticated transport characteristics, for instance, one that contains the equivalent of an npn or pnp junction and thus an amplifier, or a pnpn set up that acts as a thyristor to allow control of huge currents" Eisenberg explains to Nanowerk. "Because these devices include ion selectivity, which we know how to manipulate, chances are we should be able to selectively amplify currents carried by individual chemical species. The implications are staggering as they were when the first electronic diode was converted into an amplifying triode." | |
| "We took the approach, where amino acids residues in the selectivity filter were replaced by others, one step further by creating a second selectivity filter with a net charge of comparable magnitude but opposite sign than that of the original, first selectivity filter" Dr. Henk Miedema explains to Nanowerk. "The two filters, located in the outer membrane protein F (OmpF) pore on a distance of, on average 2.6 nm, create separated regions where either cation or anions accumulate. Our work shows that biological channels can be made into electrostatic devices. Because one or more amino acid residues at any preferred position can be substituted by others, the precision and resolution of the charge distribution (created by chemical modification of a biological pore) is unprecedented compared to those in synthetic nanochannels." | |
| Miedema, a senior scientist at the Biomade Technology Foundation in Groningen, The Netherlands, is first author of a recent research paper ("A Biological Porin Engineered into a Molecular, Nanofluidic Diode") where the Dutch team at Biomade collaborated with Eisenberg's group at Rush and the whole effort was financed by NanoNed, a nanotechnology program of the Dutch Ministry of Economic Affairs. | |
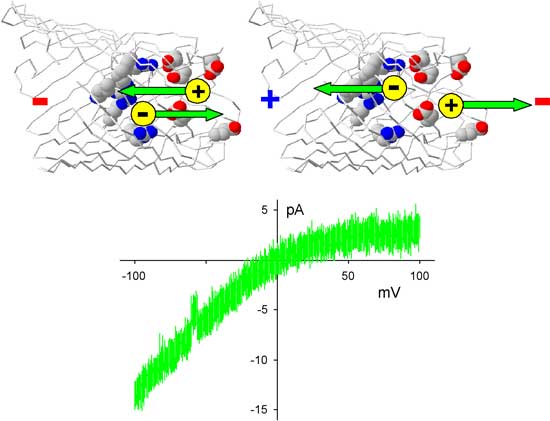 |
|
| The red colored residues in the protein are the negatively charged amino acid residues that create a region where the positively charged cations (in yellow, not at scale!) accumulate. The blue colored amino acid residues are the positively charged amino residues of the channel protein, where negatively charged anions (also in yellow) accumulate. At negative bias potentials (left part of the current-voltage plot), cations and anions move towards the junction of the two selectivity filters (=the equivalent of the np junction of a semiconductor diode) and current is flowing. At positive bias potentials, cations and anions move away from the junction, thereby creating a zone depleted of charge carriers. As a result, current is inhibited. Overall, we see a voltage-dependent current rectification. (Image: Dr. Miedema) | |
| The main motivation for this study was to point to the key role electrostatics play in the ion flow through a nanochannel. The resulting work emphasizes the similarity in physics that describes, on the one hand, the flow of electrons and holes in a doped semiconductor and, on the other hand, the flow of ions through an (charged) ion channel. | |
| "The permanent charge in the selectivity filter of an ion channel plays an analogous role in ion conduction than the role doping plays in the current of quasi-particles in a semiconductor" says Miedema (see Eisenberg's article: "Living Transistors: a Physicist's View of Ion Channels"). "If true, we should be able to introduce rectification into an otherwise non-rectifying channel by mimicking the electric field of a semiconductor diode. This was the hypothesis we tested. The observation that the current through this mutant channel not only rectifies but, in addition, shows the voltage dependence we had anticipated, makes us believe that our interpretation in terms on an ionic Nernst-Planck junction is correct." | |
| Miedema says that, due to the nature of Coulombic interactions, the role of structure in ion conduction is, in general, overrated, whereas the role of electrostatics is underrated. Our study exemplifies this statement. Even though the structure of the diode mutant was not available, its current profile is as was predicted. | |
| "Indeed" says Eisenberg, "our work suggests that the word ?conformation? found on nearly every other page of any work on proteins should be interpreted as the conformation of the electric field much more than as the shape of the protein. We suspect that changes in the shape of the electric field are the most important conformation changes in ion channel proteins. Certainly, changes in conformation of the electric field have the ability to generate a wide range of the nonlinear properties of biological systems. After all they are the only conformation changes in semiconductor diodes and transistors which perform an enormous range of nonlinear logical functions." | |
| Ion channels like the ones Eisenberg and Miedema describe may be used in nanofluidic networks composed of membranes in parallel and/or in series, all containing a specific, functionalized ion channel. Such a network resembles an electronic circuit composed of resistors, diodes, capacitors and so on. | |
| Functionalized ion channels with distinct and addressable features (e.g., ion selectivity or rectifying) might be a first step to achieve that goal, says Miedema. A recent study ("Automated Formation of Lipid-Bilayer Membranes in a Microfluidic Device") outlines a method to form phospholipid bilayers in a microfluidic device. | |
| Although there is still a long way to go, such a platform with channels reconstituted in the membranes acting as (ion selective and voltage dependent) valves would be an excellent starting point for such a network. | |
| Eisenberg and Miedema point out that there are two main challenges ahead of them. "First, to extend the OmpF pore with a third selectivity filter to create the ionic equivalent of a pnp or npn junction. Secondly, to introduce flexibility and design an np-junction that can be turned on and off by an external trigger, for instance, light. One way to achieve this is to introduce cysteines (in the originally cysteine-free OmpF) and chemically modify these introduced cysteines with light-sensitive moieties whose charge state depend on the light quality used." | |
| This exiting work is another example that shows how nanotechnology can benefit enormously from scientists' existing knowledge of the working mechanism of nanoscale biological systems. To avoid reinventing the wheel, nanotechnology can mimic and design devices inspired by nature?s nanomachines. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
