| Posted: Mar 01, 2012 | |
Direct observation of drug release from carbon nanotubes in living cells |
|
| (Nanowerk Spotlight) Carbon nanotubes (CNTs) offer a number of advantages for delivering drugs to specific locations inside the body which suggest that they may provide an improved result over nanoparticles. They have a larger inner volume which allows more drug molecules to be encapsulated, and this volume is more easily accessible because the end caps can be easily removed, and they have distinct inner and outer surfaces for functionalization. Recent research has shown the ability of CNTs to carry a variety of molecules such as drugs, DNA, proteins, peptides, targeting ligands etc. into cells – which makes them suitable candidates for targeted delivery applications (see our previous Nanowerk Spotlight: "Swallowing a nanotechnology pill"). | |
| Doxorubicin (DOX), a popular anticancer drug, is promising for use in controlled drug-release systems. Due to its aromatic structure, DOX can be attached to the side-wall of single-walled carbon nanotubes (SWCNTs) and then detach to yield free molecules in acidic conditions because of the pH-dependent stacking interaction between DOX and nanotubes. Researchers have already successfully applied a DOX-loaded carbon nanotube drug delivery system for both in vitro ("Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery") and in vivo ("Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy") cancer treatment. | |
| "The precise process of drug release from its vehicle inside the living cell is still not clear," says Da Chen, a professor in the College of Material Science and Technology at >Nanjing University of Aeronautics and Astronautics. "Understanding this process is quite important to better understand the subcellular mechanisms of drug delivery systems therapy and therefore to design new systems and controlled-release approaches. In the DOX-loaded carbon nanotube drug delivery system, DOX is supposed to release to intracellular lysosomes after being delivered into cells due to the acidic microenvironment in the lysosomes. However, thus far, the direct observation of this drug-release process inside the living cells has not been seen. Tracking this process in living cells requires the determination of the location and movement of the carbon nanotube carriers and the drug status – loading or release – simultaneously, which seems quite difficult to achieve." | |
| In recent work, Chen and his collaborators have now developed a unique two-dye labeling method to directly track the release process of DOX from carbon nanotube carriers in living cells. | |
 |
|
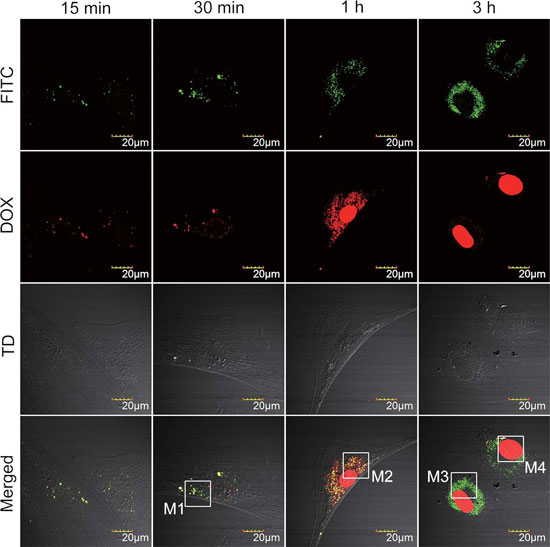
| Tracking drug release from SWCNTs inside the cell. Multichannel confocal images of endothelial progenitor cells after incubation with FITC-CS/SWCNTs/DOX for 15 min (left panel), 30 min (second panel), 1 h (third panel), and 3 h (right panel). The green fluorescence shows the location and movement of SWCNTs inside the cell and the red fluorescence shows the drug DOX. The separation of red fluorescence (DOX) from green (FITC-CS/SWCNTs) in the merged channel indicates drug release. The concentrations of FITC-CS/SWCNTs/DOX in all groups were 20 µg mL-1. M1–M4 are magnified in the figure below. (Reprinted with permission from Wiley-VCH Verlag) | |
| Reporting their work in a recent issue of Small ("Subcellular Tracking of Drug Release from Carbon Nanotube Vehicles in Living Cells"), the team demonstrates the direct observation of the time-dependent drug-release process using a laser scanning confocal microscope. Based on this observation, they propose a three-step process model for subcellular drug release from a carbon nanotube vehicle. | |
| To perform their observations, the researchers covalently conjugated a dye that exhibits bright green fluorescence to chitosan-coated carbon nanotubes. This allowed them to track the nanotube location and movement inside cells. Then the drug DOX, which is also a kind of dye that exhibits bright red fluorescence, is attached by means of π-stacking onto the side-wall of the SWCNTs. | |
| "This pH-sensitive interaction between DOX and SWCNTs should induce drug release in acidic conditions" says Chen. "Thus, from the performance of these two kinds of fluorescence – together or separated – we monitored the drug status on nanotube carriers, and also the pH change in specific subcellular organelles that triggered the release." | |
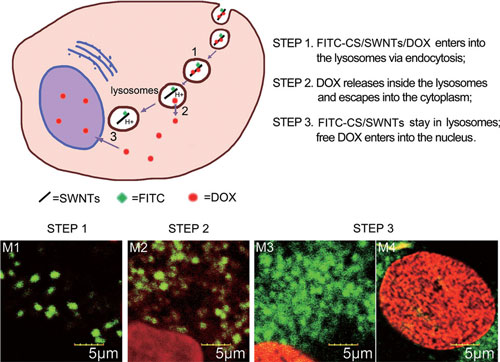
| From their observations of the subcellular behavior of drug-loaded SWCNTs, the scientists concluded the mechanism of drug release inside the cell as illustrated below: | |
 |
|
| Schematic of subcellular drug-release process. Three steps occur for DOX release from the carbon nanotube based delivery system, as illustrated in the cell diagram and respective confocal images. (Reprinted with permission from Wiley-VCH Verlag) | |
| The team says that these findings are partly consistent with the previously proposed mechanism for drug release. "Additionally, we noticed some exceptional phenomena in our experiments" says Chen. "For instance, after being internalized into the cells, SWCNTs are actively transported into a perinuclear area without obvious exocytosis, which is different from the traditional endocytosis pathway we observed previously. So the cellular metabolic pathway of SWCNTs still requires systematic study in the future." | |
| Nevertheless, the experimental establishment of drug-release mechanisms in living cells, as demonstrated in this work, may provide important insights to better understand the drug-release process and aid the future design of new drug-delivery systems. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
