| Posted: Mar 02, 2012 | |
Exploring the complexity of nanomaterial-neural interfaces |
|
| (Nanowerk Spotlight) Carbon nanotubes, like the nervous cells of our brain, are excellent electrical signal conductors and can form intimate mechanical contacts with cellular membranes, thereby establishing a functional link to neuronal structures. There is a growing body of research on using nanomaterials in neural engineering (see our Nanowerk Spotlight: "Nanotechnology to repair the brain"). | |
| Now, researchers have, for the first time, explored the impact of carbon nanotube scaffolds on multilayered neuronal networks. Up to now, all known effects of carbon nanotubes on neurons – namely their reported ability to potentiate neuronal signaling and synapses – have been described in bi-dimensional cultured networks where nanotube/neuron hybrids were developed on a monolayer of dissociated brain cells. | |
| In their work, a team of scientists in Italy, led by professors Maurizio Prato and Laura Ballerini, used slices from the spinal cords of mice to model multilayer-tissue complexity. They interfaced these spinal segments to multi-walled carbon nanotube (MWCNT) scaffolds for weeks at a time to see whether and how the interactions at the monolayer level are translated to multilayered nerve tissues. | |
| In a paper in the February 16, 2012 online edition of ACS Nano ("Spinal Cord Explants Use Carbon Nanotube Interfaces To Enhance Neurite Outgrowth and To Fortify Synaptic Inputs"), the team reports the efficacy of carbon nanotube neuron interactions to generate signals which are then translated into activity modifications. | |
| "Our findings are entirely new in that we propose that nanomaterial/cell interactions can be instructive for network behavior and for signaling even at a distance from the interface," Ballerini explains to Nanowerk. "This means that neurons are capable of sensing a nanomaterial and translate this interface into improved activity within a larger area of influence in spinal explants. This issue has been unexplored up to know." | |
 |
|
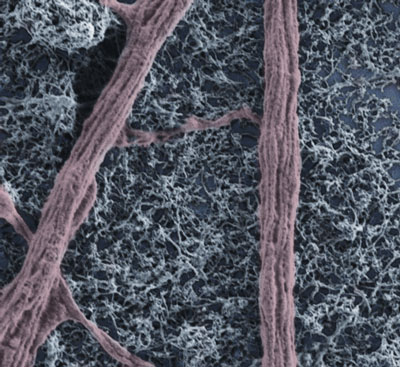
| Modified (by D.Scaini, Prato-Ballerini research group) scanning electron microscopy image of spinal explant peripheral neuronal fiber on a MWCNT substrate. (Image: Prato-Ballerini research group) | |
| According to the team, interfacing spinal cord explants to purified carbon nanotubes over a longer period (weeks) induces two major effects: First, the number and length of neuronal fibers outgrowing the spinal segment increases, associated with changes in growth cone activity and in fiber elastomechanical properties. And, secondly, the researchers point out that after weeks of MWCNT interfacing, neurons located at as far as five cell layers from the substrate display an increased efficacy in synaptic responses – which could represent either an improvement or a pathological behavior – presumably mediated by ongoing plasticity driven by the neuron/MWCNT hybrids. | |
| "We propose that these two effects rely on direct and indirect MWCNT interactions" says Ballerini: "The first being mediated by direct adhesion of outgrowing fibers to the nanostructured carbon substrate; the second by alterations in the activity of neuronal layers interfaced to the substrate, able to influence remote, although synaptically coupled, neuronal ensembles." | |
| The team says that they cannot entirely exclude that the effects on remote neurons are mediated by small amounts of detached MWCNTs, internalized by cells within the spinal explants, and not detected by routine TEM analysis. However, some of their observations seem to rule out this possibility. | |
| Going forward, the major challenge is to unravel the signal and transduction pathways involved in the detected influence of carbon nanotubes to neuronal networks. | |
| Ballerini notes that clarifying this issue will allow the future design of scaffolds and interfaces that, by means of their physical and chemical structure, replicate instructive features of the extracellular micro environment at the nanoscale. | |
| "Ongoing efforts in regenerative medicine require the development of synthetic extracellular scaffolds able to provide unique micro-environments to tissue-specific cell types" she says. "Micro- and nanoscale techniques employed to recreate interactions between cells and tissue-engineering scaffolds offer great promise in the fabrication of biological tissue constructs. These interactions are widely accepted as being essential to promote tissue and to maintain tissue function in tissue repairing processes. Thus, exploiting physical and chemical features at the nanoscale may improve next generation of transplantable devices for tissue implants." | |
| Prato and Ballerini point out that, ultimately, nanomaterial-based scaffolds will allow to investigate the ability of multilayered nervous tissue in translating adhesive interactions into network activity in regions relatively far from the interface itself, providing relevant information for the scientific community dealing with neuronal interfaces and carbon nanotubes. | |
| They note that this is important because it exploits the design of artificial micro- and nanoscale devices that cooperate with neuronal network activity, thereby creating hybrid structures able to cross the barriers between artificial devices and neurons. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
