| Posted: Mar 23, 2012 | |
Compelling evidence for silicene - the silicon analogue to graphene |
|
| (Nanowerk Spotlight) The fascination with two-dimensional (2D) materials that has started with graphene has spurred researchers to look for other 2D structures like for instance metal carbides and nitrides (see our Nanowerk Spotlight "Graphene was only the beginning; now MAX phases get two-dimensional as well"). One particularly interesting analogue to graphene would be 2D silicon – silicene – because it could be synthesized and processed using mature semiconductor techniques, and more easily integrated into existing electronics than graphene is currently. | |
| However, silicene does not seem to exist in nature nor is there any solid phase of silicon similar to graphite. Nevertheless, silicene has been predicted by theory as early as 1994 (see paper in Physical Review B: "Theoretical possibility of stage corrugation in Si and Ge analogs of graphite") and, with the explosive research going into graphene, there has been growing interest in this field over the past few years. | |
| The issue with theoretical calculations is that they calculate the total energy of a system like silicene and thus predict whether it is energetically stable or not. What theory cannot predict is whether such a material can also occur naturally or be formed in in the lab. | |
| "As a matter of fact, silicene has not been observed in nature nor is there any solid phase of silicon similar to graphite," Dr. Patrick Vogt, a researcher in the Experimental Nanophysics and Photonics group at Technical University of Berlin, tells Nanowerk. "As a consequence, pure 2D silicene layers cannot be generated by exfoliation methods as in the case of graphene and more sophisticated methods have to be considered for the growth of silicene." | |
| In recent work (see accepted paper in Physical Review Letters: "Silicene: Compelling Experimental Evidence for Graphenelike Two-Dimensional Silicon"), though, Vogt and his collaborators Guy LeLay from CNRS-CINaM in Marseille, Paola DePadova from CNR-ISM in Rome, and colleagues from their groups as well as from the Synchrotron SOLEIL in Saint Aubin, have presented the first clear evidence for the synthesis and thus the existence of silicene – a two-dimensional material, with a honeycomb-like arrangement of silicon atoms. | |
 |
|
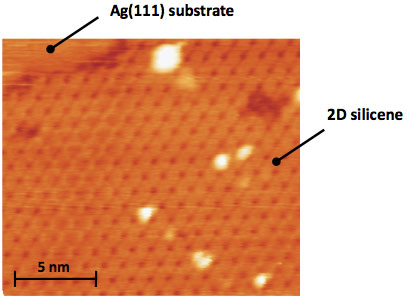
| STM image of a 2D Si-layer on Ag(111). Clearly visible is the honeycomb-like structure. (Image: Dr. Vogt, TU Berlin) | |
| "The most important motivation for our work was to demonstrate the existence of silicene by growing it on templates that do not react with the silicon atoms, such as Ag(111) surfaces," explains Vogt. "In the literature, there has been only one publication ("Epitaxial growth of a silicene sheet") before which claims the synthesis of silicene on Ag(111) based on STM measurements only. We explain in our manuscript why we are convinced that these results only refer to measurements of a clean Ag(111) surface. This is not the first time in research that STM measurements are misleading in order to find an atomic structural model. Furthermore, the pure Ag(111) surface can sometimes appear as a honeycomb structure due to an electronic effect caused by the STM tip." | |
| In this respect it was extremely important for the research team to be clear about what they could consider to be a proof for the existence of silicene. They concluded that this proof can only be found if they could show that the silicene layer formed on top of the silver surface shows a 2D growth mode; has the structural and chemical aspects expected for silicene; has a reasonable Si-Si distance between neighboring atoms in the layer; has an electronic dispersion resembling that of relativistic Dirac fermions; shows a Fermi velocity expected for such a 2D system; and, finally, is energetically stable. | |
| Such an ambitious task can only be achieved by combining several experimental and theoretical techniques. In their paper, the scientists provide compelling evidences, from both structural and electronic properties, for the synthesis of epitaxial silicene sheets on a silver (111) substrate, through the combination of scanning tunneling microscopy and angular-resolved photoemission spectroscopy in conjunction with calculations based on density functional theory. | |
| Vogt points out that the synthesis of silicene opens up interesting perspectives since silicene – with nontrivial electronic structure and a much larger spin-orbit coupling of 1.55 meV than in graphene – is predicted to exhibit a quantum spin Hall-effect in an accessible temperature regime. | |
| "At the same time silicene also shows important advantages with respect to graphene, for example as it can easier be combined with silicon-based electronics and logic devices," he says. | |
| One of the main challenges for the near future is to grow silicene on other materials than metals, e.g. semiconductors or insulators. This would allow to directly access properties such as the conductance and give researchers a first idea on the possibilities for technological applications. | |
| "At the same time, we will also focus on the separation of the silicon from the substrate to obtain it as a free-standing system," says Vogt. "Another important aspect will be to look for other 2D material systems such as germanene, the graphene/silicene analogous for germanium and for combined or multi-layer 2D systems." | |
| This work was financially supported within the project 2D-NANOLATTICES of the Future and Emerging Technologies (FET) program within the 7th framework program for research of the European Commission and the Deutsche Forschungsgemeinschaft (DFG) under grant number VO1261/3-1. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
