| Posted: Jul 24, 2012 | |
The cellular response to nanomaterials depends on the environment |
|
| (Nanowerk Spotlight) Understanding and controlling the interaction of nanomaterials with the human body is vital for the safe and effective development of nanotechnology. The overall response of the body to a nanomaterial results from a series of cell-nanomaterial interactions. In studying cell-nanomaterial interactions, researchers often assume that the form that a nanomaterial takes when it comes into contact with a cell is the same as the form it had following synthesis. This is not always a valid assumption. Following synthesis, many nanomaterials have high surface energies. When such a nanomaterial enters a physiological environment, it attempts to lower its surface energy by adsorbing surrounding biomolecules including proteins, lipids, small molecules, saccharides, and nucleic acids. Provided the adsorbed biomolecules are retained, they define a new interface between the nanomaterial and its surroundings. It is this interface that mediates subsequent cellular association and response. | |
| Biomolecule adsorption to nanomaterials is usually studied from physiological fluids with suspended biomolecules. Examples include blood serum/plasma, pulmonary surfactant, and synovial fluid. However, until now the amount of those molecules has not been considered relevant to the study. In a recent article appearing in ACS Nano ("Effects of the Presence or Absence of a Protein Corona on Silica Nanoparticle Uptake and Impact on Cells"), Drs. Anna Salvati, Kenneth Dawson, and their colleagues at the University College in Dublin, Ireland, show that if nanoparticles are exposed directly to cells in the absence of suspended biomolecules, the nanoparticles will extract biomolecules directly from cells themselves. | |
| In their experiments, the team exposed silica nanoparticles to cells in two sets. One set was introduced into cell culture media that was supplemented with the usual concentration of fetal bovine serum, and the other into media that had no serum additives. They then incubated both sets of particles with a lung cancer cell line and measured particle uptake kinetics and cell adhesion. Nanoparticles treated under both conditions associated with cells. However, the particles that were incubated in media alone associated to a much greater extent than those that were first incubated in serum. This indicates that the affinity of the bare nanoparticle surface to the cell is much higher than the affinity of an equivalent surface that is coated with serum proteins. Similar observations are reported before for other systems, where it was also found that uptake under serum-free conditions is higher. | |
| Dawson and his colleagues suspected that the cell association of the uncovered nanoparticles was mediated by direct interaction between the nanoparticle and the cell surface. To test whether the nanoparticles were extracting cellular proteins, they isolated extracellular particles after incubation with cells and identified the adsorbed proteins using mass spectrometry. Not surprisingly, proteins isolated from particles in the serum-containing media were almost exclusively serum-derived. However, proteins isolated from particles incubated in serum-free media were entirely cell-derived. This shows that if biomolecules are not provided in the media, then the nanoparticles will find them from other sources, namely cells. | |
 |
|
| Schematic illustrating the difference in cellular and biomolecular interactions between silica nanoparticles in serum-containing and serum-free media. (Reprinted with permission from American Chemical Society) | |
| “When the nanomaterial is put in contact with a physiological environment, it is given a menu of possible biomolecules to adsorb” explains Dawson. “It will essentially go shopping for the biomolecules that it wants. Over time, it will exchange with the environment until it finds the things that it really likes most. If you don’t give it enough biomolecules in the form of serum, it will extract components from the cells themselves.” | |
| The same silica nanoparticles exposed to cells in the two different conditions had different cellular responses as well. Most of the serum-coated particles were taken up within vesicles in the cell cytoplasm and produced no overt signs of toxicity. In contrast, the particles without a serum coating adhered to the cell surface to a greater extent, were present in vesicles, and some were also found free-floating in the cytoplasm. Exposure to particles in absence of serum significantly decreased cell viability and caused cells to take on a rounded morphology that is indicative of cell death. Dawson believes that cell death from uncoated particles is the result of strong interactions between the particle surface and the cell surface, which may damage the cell membrane, and/or initiate aberrant signaling cascades. When serum proteins are adsorbed to the nanoparticles, they ‘passivate’ the surface and limit direct nanomaterial-cell interactions. | |
| When considering the early interactions of a nanomaterial with a cell, Dawson points out that one cannot think of the nanomaterial alone. Instead, one must think of the nanoparticle and its adsorbed biomolecules as a fundamental unit. | |
| “It is impossible to define a nanoparticle without the environment that it is in. We feel that this is a canonical statement. It says that it is not the intrinsic properties of the particle alone, but the extrinsic properties that it acquires by contact with the environment that matter. So it means that we have to leave aside the concept that the nanomaterial and its surface alone is enough to specify the likely outcome.” | |
| The same nanoparticle can take on many different forms by acquiring unique biomolecule coronas that depend on the particular environment that it encounters. Depending on the composition of the corona, the nanoparticle may elicit very different responses from the cells that it meets. | |
| Dawson believes that researchers must pay closer attention to the composition of the biomolecular environment surrounding the particles and cells when performing in vitro experiments. In other words, it is as important to consider the composition of the biomolecules in the media as it is to consider the chemical nature of the nanoparticle and the cell type. | |
| “What’s absolutely clear is that depending on the type of dispersion that you make up, whether you add 10% serum or 20% serum, you get different levels of cell uptake” says Dawson. “Indeed, you get different levels of damage as well. It is therefore not meaningful to say that the nanoparticle is or is not toxic in that simplistic way. You can make a material toxic if you really want to make it toxic. You can make many materials damage cells simply because these have high surface energy. However, in a realistic physiological environment, part of the particle surface is covered and so that kind of damage would not be applicable.” | |
 |
|
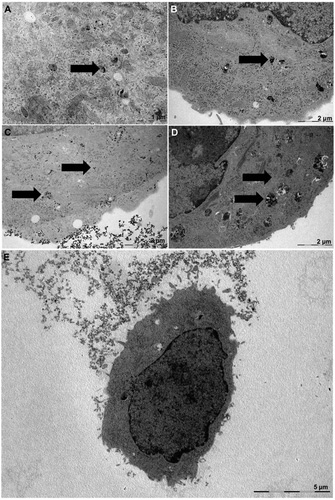
| Transmission electron microscopy images of lung cancer cells exposed to silica nanoparticles in serum-containing (A and B) and serum-free media (C and D). Low-magnification image of a cell after exposure to nanoparticles in serum-free media. (Reprinted with permission from American Chemical Society) | |
| Dawson further suggests that failure to adequately consider the biomolecular corona in the past may be, at least partially, responsible for the widely-reported inconsistencies in cell response between different studies and the poor correlation between in vitro and in vivo experiments. | |
| “It is possible that some of the inconsistencies reported in the past are because of factors that have not been properly controlled. If we both have similar systems, and you use twice the amount of serum as me, we could arrive at completely different results. Alternatively, we may both use the same volume of protein. Then I look and realize I’ve used 10 times the total nanoparticle surface area that you have. There may not be enough protein in my solution to produce the same corona that you had in yours. You can immediately see that if you don’t control these factors, there is no reason to expect the results to agree. If we’re not controlling these factors, we should not be too shocked about the lack of reproducibility of our results.” | |
| If results from different studies are to be compared, it will be necessary to standardize the experimental conditions and their detailed reporting in the future. | |
| “We have to set up agreements within the community” says Dawson. “We have to work on the same systems if we’re going to compare results. That’s the most basic point. A science is not a science until you control all of the relevant parameters, and you report them and other people can reproduce them. We simply haven’t been controlling one of the parameters. There is nothing wrong with reporting that silica or some other substance that is ‘naked’ damages cells, but it is entirely obvious that it would. There’s a huge surface energy that is coming into contact with the cells. But we should be sure that we explain what it is that we’re doing.” | |
| A deeper concern is that these types of reports could obscure the real issues of nanoparticle safety, leading people to look for danger where there is not any. | |
| Besides standardization and reporting, it will also be necessary to find in vitro cell culture conditions that accurately reflect the in vivo environment. Cell experiments with nanomaterials often use 10% fetal bovine serum as an additive in the cell culture media. This condition is inherited from molecular biology experiments, not because it necessarily reflects the physiological environment that exists in vivo. | |
| “The real reason we put proteins into the media was historically to feed the cells, so there was no attempt being made to mimic the in vivo situation” explains Dawson. “Because of that, there was no necessity to worry about the details of how much you put in. In molecular studies, the serum is really a source of amino acids and growth factors. It doesn’t even matter the species of the serum. That environment doesn’t represent what we would be seeing in vivo. The idea of taking the most extreme case of media without serum is that there you can see quickly that nanoparticles, in many cases, are physically damaging the cells, and that’s entirely unrealistic. You might then add 10% serum protein, but that’s not relevant either. The relevant parameter is the total surface area of the nanoparticle dose you administer. Depending on the dose you add of the particles and the volume you’re working with, you may not have enough protein, even at 10% serum, to properly cover the particles. You could still see damage there that is not appropriate, not close to reality. In this case, there is an extremely high energy surface that is being brought into contact with delicate biological structures - something that is never going to happen in real life.” | |
| Ultimately, the results of this study show that the early cellular response to a nanomaterial is intimately tied to the composition of the surrounding biomolecular environment. The composition of the environment determines the protein corona formed on the nanomaterial, which, in turn, influences its interactions with the surroundings. One must consider carefully the dispersion medium in order to understand the formation of the protein corona and the downstream cellular effects. Despite the mounting evidence supporting the importance of environmental adsorbates on nanomaterial behaviour in a biological system, it remains a relatively unexplored and underappreciated issue in nanotechnology. Raising awareness about this issue will undoubtedly take time. However, it will be required if the interaction of nanomaterials with cells and the body is going to be fully and accurately understood at a mechanistic level. | |
| By Carl Walkey, Integrated Nanotechnology & Biomedical Sciences Laboratory, University of Toronto, Canada. | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
