| Posted: Sep 18, 2007 | |
Nanotechnology diamond ice coatings could improve knee prostheses and solar cells |
|
| (Nanowerk Spotlight) There is a huge demand for medical implants for almost every body part you can think of. As we have reported here before, the market for medical implant devices in the U.S. alone is estimated to be $23 billion per year and it is expected to grow by about 10% annually for the next few years. Implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, tissue and spinal orthopedic implants, hip replacements, phakic intraocular lenses and cosmetic implants will be among the top sellers. | |
| Current medical implants, such as orthopedic implants and heart valves, are made of titanium and stainless steel alloys, primarily because they are biocompatible. Unfortunately, in many cases these metal alloys with a life span of 10-15 years may wear out within the lifetime of the patient. | |
| With recent advances in industrial synthesis of diamond and diamond-like carbon film bringing prices down significantly, researchers are increasingly experimenting with diamond coatings for medical implants. On the upside, the wear resistance of diamond is dramatically superior to titanium and stainless steel. On the downside, because it attracts coagulating proteins, its blood clotting response is slightly worse than these materials and the possibility has been raised that nanostructured surface features of diamond might abrade tissue. | |
| That's not something you necessarily want to have in your artificial knee or hip joints (although some of the currently used implant materials cause problems as well). | |
| Researchers have now run simulations that show that thin layers of ice could persist on specially treated diamond coatings at temperatures well above body temperature. The soft and hydrophilic ice multilayers might enable diamond-coated medical devices that reduce abrasion and are highly resistant to protein absorption. | |
| Due to its excellent biocompatibility, diamond has been called the "Biomaterial of the 21st Century".If you want to read up on this topic, Robert Freitas covers the Biocompatibility of Diamond – together with other nanomaterials – extensively and with numerous links and references in his online nanomedicine book. There is a chapter dedicated to Biocompatibility of Diamond-Coated Prostheses. | |
| "Low-temperature and short-ranged interfacial ordering of water has been previously predicted and observed on a variety of other planar substrates, including muscovite mica, platinum, chlorine-terminated silicon, quartz, and graphite" Alexander Wissner-Gross tells Nanowerk. "However, in order to stabilize ice coatings with the nanometer-scale thicknesses relevant to macromolecular adsorption, at temperatures relevant to in vivo application, a novel substrate is needed." | |
| In his latest paper, written together with Prof. Efthimios Kaxiras, the Gordon McKay Professor of Applied Physics & Professor of Physics at Harvard University, Wissner-Gross describes exactly such a substrate ("ARTIKEL")>"Diamond stabilization of ice multilayers at human body temperature"). | |
 |
|
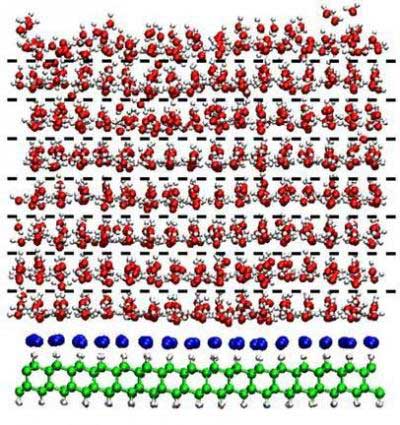
| Water molecules (red and white) form orderly layers of ice on top of a layer of diamond (green) treated with sodium atoms (blue) in a simulation showing that such ice layers could persist well above body temperatures. Potentially, high temperature ice could make diamond coatings more suitable for implanted joints, heart valves, and other medical devices. (Image: Alexander D. Wissner-Gross and Efthimios Kaxiras) | |
| The scientific core of the Harvard scientists' findings is that chemically-modified diamond is the strongest surface stabilizer of ice yet discovered. According to their simulations, diamond with an atomically-thin treatment of sodium can prevent ice films a few nanometers thick – the size of a very small protein – from melting at temperatures beyond human body temperature. You might call it 'warm ice.' | |
| "We think the result is exciting from a nanotechnological perspective because it opens the door to a new class of atomically-engineered surfaces with novel physical properties, such as long-range ice stabilization" says Wissner-Gross. | |
| The physicists arrived at their result by using a computer simulation based on molecular dynamics. Direct simulations of the freezing phase transition are challenging, says Wissner-Gross so the focus was made instead on understanding the stability of initially crystalline ice layers. Many hypothetical models have been developed in order to discover the structure of water and Wissner-Gross and Kaxiras chose to use the TIP4P/Ice water model in order to reproduce the melting temperature of ice. | |
| Modeling the motion of water atoms sitting on top of a sodium-diamond surface at different temperatures over long time periods resulted in the surprising findings that ice layers could persist on the treated diamond up to temperatures of 108 degrees Fahrenheit (42° Celsius), and in some circumstances could remain frozen beyond the boiling point of water. | |
| The researchers think that the main application of their research will be for coating medical implants. | |
| "In particular, we have demonstrated a new, potentially biocompatible, diamond nanocoating that could make human joints completely scratch- and wear-resistant" says Wissner-Gross. "This is a major finding for patients who suffer from arthritis and other diseases that require joint replacement surgery. Diamond has long been an attractive material for scratch-free coatings and is becoming inexpensive enough for biomedical applications, such as bone and enamel coatings. Our new research demonstrates that, with the proper chemical modification, diamond might be made extremely bio-friendly by shielding itself with a thin layer of ice at human body temperature." | |
| In addition to the medical application, this novel physical effect may prove useful in making solar energy more economically viable. In particular, the effect may enable solar thermal collectors to compensate for variability in the insulation supply by locally buffering their thermal output using hot water sequestered on such diamond surfaces. Because diamond can elevate the solid-liquid transition of water close to its liquid-vapor transition, 50% more latent heat could be stored near the melting point than with a traditional heat exchanger based on the solid-liquid transition. This water-based approach to small-scale solar energy storage and buffering would have the additional advantage of being more environmentally friendly than lithium-ion batteries or organic phase-change materials. | |
| "I see surface physics as a tremendously interesting field going forward" says Wissner-Gross. "The main trend I see is toward adding more functionality to everyday surfaces, whether by nanostructuring (in this case) or other mechanisms. While synthesizing all the surfaces we can imagine will always be a challenge, I think the field is up to the challenge." | |
| A short film that the researchers made from of some of their simulations was a finalist in the 2007 Materials Research Film Festival: | |
| The work also earned Wissner-Gross the 2007 Dan David Prize Scholarship from Tel Aviv University and the 2007 Graduate Student Silver Award from the Materials Research Society. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
