| Posted: Sep 24, 2007 | |
Nanotechnology optimizes catalyst systems |
|
| (Nanowerk Spotlight) Back in the early 1800's it was observed that certain chemicals can speed up a chemical reaction - a process that became known as catalysis and that has become the foundation of the modern chemical industry. By some estimates 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture. Catalysis is the acceleration of a chemical reaction by means of a substance, called a catalyst, which is itself not consumed by the overall reaction. The most effective catalysts are usually transition metals or transition metal complexes. An everyday example of catalysis is the catalytic converter in your car which is used to reduce the toxicity of emissions from your car's engine. Here the catalysts are platinum and manganese which for instance convert harmful nitrogen oxides into harmless nitrogen and oxygen. Since catalysts provide a surface for the chemical reaction to take place on, nanoparticles with their extremely large surface area have become much researched as catalysts (as particles get smaller the larger their surface to volume ratio becomes). Especially in heterogeneous catalysis - where the catalyst is in a different phase (ie. solid, liquid and gas) to the reactants, and that is largely influenced by surface properties - use of nanoscale catalysts opens up a number of possibilities of improving catalytic activity and selectivity. Unfortunately, heterogeneous catalysts supported on a carrier prepared using traditional methods (e.g., impregnation) suffer from a number of problems, such as particle aggregation during preparation, sintering during use (especially at high temperatures), and catalyst leaching because of solvent or pressure drop. This is associated with the poor contact of the catalyst particle with the support surface. A new method of catalyst preparation coming out of Singapore may offer a new concept for catalyst optimization. | |
| "We rationally designed a nanostructured catalyst with ruthenium nanoparticles sandwiched in the pore walls of carbon" Dr. Xiu Song Zhao explains to Nanowerk. "Such a nanostructure on one hand can significantly minimize particle aggregation, movement and leaching, and on the other ameliorate the metal-support interaction, thus leading to a great catalytic performance in terms of catalytic activity and lifetime." | |
| Since the discovery of ruthenium as a catalyst for hydrogenation reactions, ruthenium catalysts have already been widely used in the chemical, petrochemical, food, and pharmaceutical industries, and in energy-conversion technologies. What is new in Zhao's work is that catalyst nanoparticles are embedded in the pore walls of a solid support instead of dispersing them on the surface. In this way, the catalyst can be stabilized. | |
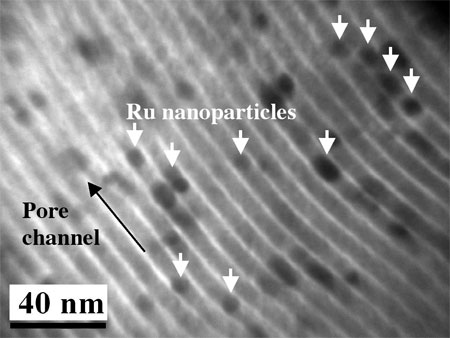 |
The TEM image clearly shows sandwiched ruthenium nanoparticles in porous carbon. (Image: Dr. Zhao, National University of Singapore) |
| The preparation is based on a template-synthesis strategy. First, catalyst nanoparticles dispersed on the pore surface of a hard template (e.g., zeolite Y and mesoporous SBA-15) are prepared. Then, the pores of the template are filled with a carbon precursor followed by carbonization. Finally, the template was removed by using a HF solution to yield a porous carbon with the catalyst nanoparticles incorporated in the carbon matrix. | |
| Zhao points out that one of the most important things is to ensure that the catalyst particles are exposed to the pore channels and thus available for catalytic reactions. "Normally, the catalyst is covered with a layer of carbon when the chemical vapor deposition (CVD) method is used for carbon infiltration" he says. "Our present work solved this problem by allowing catalyst particles to grow in the pores of the template before carbon infiltration. Eventually, the size of the catalyst particles is determined by the pore size of the template. The contact spots of the catalyst particle with the pore surface of the template are the active sites of the final carbon-carried catalyst." | |
| Thus a number of advantages of such ruthenium/carbon nanostructured catalysts over those prepared using conventional methods can be anticipated, such as firm fixation of the ruthenium nanoparticles in the carbon matrix, no aggregation of the ruthenium nanoparticles, no pore blocking, an extremely intimate contact between the metal and support, controllable ruthenium nanoparticle size, and tailorable pore size of the support. | |
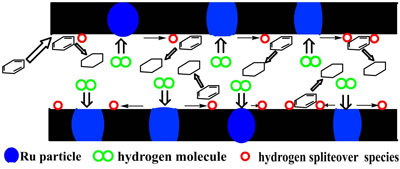 |
This scheme illustrates catalytic hydrogenation of benzene enhanced by hydrogen spillover promoted by the ruthenium-carbon nanostructured catalyst (Image: Dr. Zhao, National University of Singapore) |
| Zhao's team is now exploring the role of such sandwiched nanostructures in applications such as ammonia synthesis, selective hydrogenation, and hydrogen storage facilitated by hydrogen spillover. | |
| "The further investigation on hydrogen spillover enhanced by such sandwiched ruthenium-carbon nanostructure is really needed" says Zhao "and will be helpful for us to design the newly and practically industrial heterogeneous ruthenium catalysts." | |
| Ultimately, such a catalyst as described in the researchers' recent paper ("Sandwiched Ruthenium/Carbon Nanostructures for Highly Active Heterogeneous Hydrogenation") may find applications in selective hydrogenation, storage of hydrogen, and methanol electrooxidation for fuel cell technology. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
