| Posted: Oct 30, 2012 | |
Sneaking drugs into cancer cells with carbon nanotubes |
|
| (Nanowerk Spotlight) The ideal drug carrier may be something out of science fiction. In principle, it is injected into the body and transports itself to the correct target, such as a tumor, and delivers the required dose at this target. This idealized concept was first proposed by Paul Ehrlich at the beginning of the 20th century and was nicknamed the 'magic bullet' concept. With the advent of nanotechnology and nanomedicine this dream is rapidly becoming a reality. | |
| A previous Nanowerk Spotlight written by Dr. Tamsyn Hilder from the Nanomechanics Group at the University of Wollongong illustrates this concept ("Nanotechnology's 'magic bullet'"). Hilder, together with Prof. James Hill, also published a review which describes the potential advantages of carbon nanotubes ("Modeling the Loading and Unloading of Drugs into Nanotubes"). | |
| In this review, the authors write that "functionalized carbon nanotubes (CNTs) might be able to target specific cells, become ingested, and then release their contents in response to a chemical trigger. This will have significant implications for the future treatment of patients, particularly those suffering from cancer, for whom presently the nonspecific nature of chemotherapy often kills healthy normal cells." | |
| A group of researchers has now essentially achieved this goal. Reporting their work in the October 3, 2012 online edition of Nano Letters ("Trojan-Horse Nanotube On-Command Intracellular Drug Delivery"), a team led by John Marshall, a professor in the Department of Molecular Pharmacology, Physiology and Biotechnology at Brown University, and Professor Jimmy Xu has encapsulated drugs inside carbon nanotubes for drug delivery and shown that these drugs can be released 'on command' by inductive heating with an external alternating current or pulsed magnetic field. | |
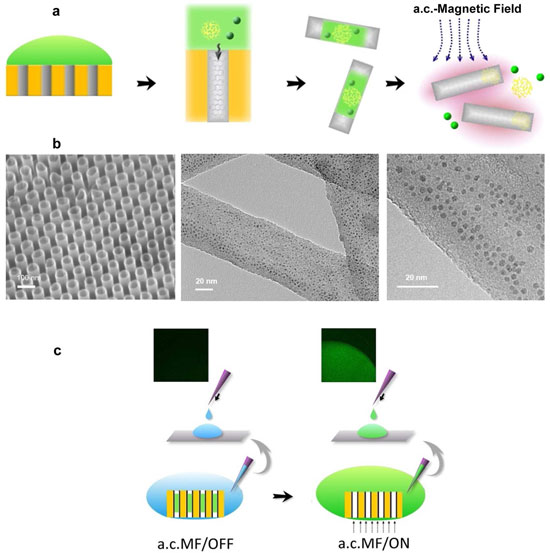 |
|
| Loading and on-command release of Trojan-Horse nanotubes by the induction heating of CNTs. (a) Schematic illustration of filling QDs/ gelatin/drug into nanotubes. The payload is loaded into the nanotubes array by the vacuum suction at the back side of the template followed by the release of Trojan-Horse nanotube from template by chemical dissolution of AAO template. Finally, the payload is released from the Trojan-Horse nanotubes by exposing Trojan-Horse nanotubes to the a.c. magnetic field. (b, left) SEM image of highly ordered carbon nanotube array. (center) TEM image showing the high loading yield of QDs into nanotubes. (right) A magnified TEM micrograph showing the QDs with diameter ∼4 nm are inside the nanotubes. (c) QD loaded nanotube array immersed in water and subjected to an a.c. magnetic field for 30 min. Thereafter, the solution was placed onto a glass slide and visualized by epifluorescence microscopy. (left) In the absence of the a.c. magnetic field induction, luminescence was not observed. (right) a.c. magnetic field induction results in a significant green luminescent signal corresponding to the QD luminescence emission wavelength. | |
| "The important advance is that we were also able to encapsulate drugs that are fairly insoluble," Marshall tells Nanowerk. "For example, we encapsulated the C6-ceramide, which can dramatically increase the efficacy of many chemotherapeutic agents including Taxol, doxorubicin and histone deacetylase inhibitors (HDACi). Unfortunately, the systematic use of C6-ceramide is limited because of its hydrophobicity and precipitation as fine lipid suspensions when administered in aqueous solutions. Other approaches, including nanoliposomes have so far been unsuccessful, as high concentration of nanoliposome ceramide was necessary to achieve anti-tumor effects." | |
| For their work, the team synthesized CNTs with a relatively large inner diameter (∼40 nm) in an array with a perfectly aligned vertical orientation. They then opened both ends of the nanotubes with either a mechanical or chemical treatment. By depositing droplets of the drug solution on top of the vertically aligned nanotube array and applying vacuum suction at the bottom the CNTs were then loaded with the desired drug. | |
| The drug solution was mixed with a temperature-sensitive hydrogel which, due to the surface tension and viscosity, keeps the gel-drug payload inside the CNTs and also prevents external water from entering the nanotube and thereby displacing the drug payload. | |
| "Our template-synthesized carbon nanotubes are intrinsically conductive but possess higher electrical resistivity than those synthesized by arc-discharging," explains Marshall. "This property is beneficial for our purpose as the electrical resistivity will generate eddy currents and consequential resistive heat via magnetic field induction." | |
| The unloading of the molecular drug cargo can be controlled by external stimulation of the CNTs with a small a.c. magnetic field pulse (which has no detectable adverse effect on healthy cells), which can reach deep into any part of the body to generate inductive heat (which also is a potential new strategy for triggering on-command drug release from heat-sensitive nanomaterials). | |
| Marshall notes that for most nanoparticles, the drugs are attached to the outside of the structure or blended into the biodegradable polymer matrix, which can result in drug degradation over the delivery process and produce off-target toxicity. | |
| "The major advantages of carbon nanotubes are that they provide a protective environment for the drug and toxic chemotherapeutic drugs can now be used at 100-fold lower dose, which will greatly reduce drug side-effects," he says. | |
| During in vitro experiments the team tested the ability of C6-ceramide and Taxol to kill three different pancreatic cancer cell lines and found that combining these two agents at a relatively low dose potently produced cell death. They then incubated the same lines of pancreatic cancer cells with CNTs loaded with this drug combination. | |
| The loaded CNTs, which had been taken up by the cancer cells, could then be opened with a 30-minute application of a 25 kHz a.c. magnetic field. As a result, the encapsulated drugs were released inside the cells which in over 70% of the cells led to cell death. | |
| Marshall points out that without magnetic field induction, the cells had a viability of almost 98%, indicating the exceptional stability of encapsulated drugs within the CNT throughout the 48-hour long incubation. | |
| People have been working with similar approaches to sneak drugs into tumors. Previously metal halides were encapsulated into CNTs, but this process involves a high-temperature (900 °C) molten-phase loading, which is unsuitable for biologics and most drugs. | |
| "We needed a new approach to deliver very toxic drugs to tumor cells and avoid harming healthy cells," says Marshall. It was Jimmy Xu's lab here at Brown who engineered these nanotubes and when he contacted my lab I immediately realized the potential in cancer treatment." | |
| Having demonstrated that their approach works in vitro, the next challenge for the scientists is to show it also works in whole animal studies and, of course, eventually people. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
