| Posted: Dec 12, 2012 | |
Nitrogen-doped graphene is an efficient catalyst for fuel cells |
|
| (Nanowerk Spotlight) Fuel cells are able to convert chemical energy to electrical energy with little pollutant emission and high energy conversion efficiency. Despite these advantages, the performance of fuel cells depends largely on the oxygen reduction reaction (ORR) – the process that breaks the bonds of the oxygen molecules – which is substantially affected by the activity of the cathode catalyst. | |
| In the ORR process, oxygen functions as an electron sink, stripping electrons from hydrogen fuel at the anode and creating the electrical pull that keeps the current running through electrical devices powered by the cell. This reaction requires a catalyst to work – with platinum so far the most popular one. | |
| Since the sluggish kinetics of ORR is the major factor impeding large-scale application of fuel cells, most research focuses on developing efficient catalysts for ORR. | |
| "The ORR process can occur in a four-electron pathway to directly produce water or a less efficient two-electron pathway involving the formation of hydrogen peroxide as an intermediate," Lifeng Dong, a Taishan Scholar Overseas Distinguished Professor at Qingdao University of Science and Technology (QUST) and an Associate Professor in the Department of Physics, Astronomy, and Materials Science at Missouri State University (MSU), explains to Nanowerk. "Platinum serves as the most efficient catalyst for the ORR in a direct four-electron pathway, yet its unstable catalytic activity, poor durability, and high cost have limited the commercial prospect of fuel cells." | |
| Consequently, a number of techniques have been explored to enhance catalytic activity and to reduce the usage of platinum. | |
| Recently, more and more research groups have demonstrated that graphene has great potential as a supporting material to efficiently disperse catalyst particles. In 2010, for instance, Dong's group reported in Carbon ("Graphene-supported platinum and platinum–ruthenium nanoparticles with high electrocatalytic activity for methanol and ethanol oxidation") that, in comparison to the widely-used Vulcan XC-72R carbon black catalyst supports, graphene-supported platinum and platinum-ruthenium nanoparticles demonstrate enhanced efficiency for both methanol and ethanol electro-oxidations. | |
| In new work, reported in the December 4, 2012 online edition of the International Journal of Hydrogen Energy ("Nitrogen-doped graphene as catalysts and catalyst supports for oxygen reduction in both acidic and alkaline solutions"), Dong and his group demonstrate high electrocatalytic activity of nitrogen-doped (N-doped) graphene synthesized by a solvothermal method in alkaline solution and its ability to improve the performance of platinum and platinum-ruthenium nanoparticles as supporting materials in acidic solution. The findings resulted from a collaborative research project between QUST and MSU. The paper was first-authored by Jincheng Bai, a graduate student in the Dual Master’s Degrees Program in Materials Science between QUST and MSU. | |
 |
|
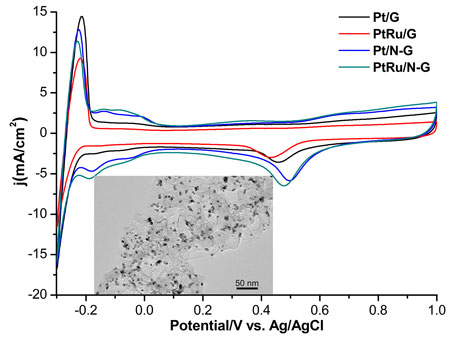
| Cyclic voltammograms for various catalysts in O2-saturated 0.1 M HClO4 aqueous solution at 50 mV/s. Inset: transmission electron microscopy image of N-doped graphene-supported Pt nanoparticles. G: graphene; N-G: N-doped graphene. (Image: Dr. Lifeng Dong, Qingdao University of Science and Technology and Missouri State University) | |
| "In comparison to graphene, N-doped graphene demonstrates higher electrocatalytic activity in both acidic and alkaline solutions," says Dong. "Nitrogen-doped graphene can act directly as a catalyst to facilitate four-electron oxygen reductions in alkaline solution and two-electron reductions in acidic solution. However, when used as catalyst supports for platinum and platinum-ruthenium nanoparticles, nitrogen-doped graphene can contribute to four-electron oxygen reductions in acidic solution, yet demonstrate much slower reaction kinetics in alkaline solution. | |
| "Our findings conclude that nitrogen-doped graphene can be developed as an efficient catalyst for oxygen reductions to replace the use of precious platinum catalysts in alkaline solution but not in acidic solution." | |
| Graphene, a mono-atomic sheet of hexagonally arranged carbon atoms, has exhibited excellent electrical conductivity and extremely high specific surface area (2600m2/g) and therefore, demonstrates its potential as a supporting material for catalyst particles. | |
| On the other hand, nitrogen and other elements have recently been successfully doped into graphene sheets (read more: "Comparing fundamental techniques for doping graphene sheets"), and N-doped graphene has demonstrated high catalytic activity for ORR via a direct four-electron pathway in alkaline solution and showed the potential to replace noble metals as electrocatalyst. | |
| Nevertheless, prior to this work, no systematic investigation of N-doped graphene, used as either a catalyst or a catalyst support for ORR in both acidic and alkaline solutions, has been published | |
| In their study, the team synthesized graphene and N-doped graphene by a solvothermal method and studied their electrocatalytic activity as catalysts and catalyst supports in both acidic and alkaline solutions. | |
| These experiments demonstrate that, for the development of high efficiency and low cost hydrogen fuel cells and direct methanol/ethanol fuel cells, N-doped graphene offers desirable electrocatalytic characteristics as catalysts in alkaline solution and as catalyst supports for platinum and platinum-ruthenium catalysts in acidic solution. | |
| The researchers are now exploring how the addition of platinum and platinum-ruthenium nanoparticles affects catalytic activity of graphene and N-graphene. They are also investigating the interfacial structures between nanoparticles and supporting materials. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
