| Posted: Jan 07, 2013 | |
Metal oxide based breath nanosensors for diagnosis of diabetes |
|
| (Nanowerk Spotlight) Human breath contains a number of volatile organic compounds (VOCs). An accurate detection of a specific VOC – i.e., a biomarker for a particular disease – in the exhaled breath, can provide useful information for diagnosis of various diseases. For example, acetone, H2S, ammonia, and toluene can be used as biomarkers for evaluating diabetes, halitosis, kidney malfunction, and lung cancer, respectively. Consequently, breath analysis has been recognized as an increasingly accurate diagnostic method to link specific gaseous components in human breath to medical conditions and exposure to chemical compounds. Sampling breath is also much less invasive than testing blood, can be done very quickly, and creates as good as no biohazard waste (see this review on breath analysis as a tool for assessing environmental exposure: "This will take your breath away"). | |
| The critical advantage of exhaled breath analysis is that it allows for non-invasive disease diagnosis. For this reason, several techniques such as gas chromatography/mass spectroscopy and optoelectronic analysis have been adopted to detect sub-ppm level VOCs in exhaled breath. However, these techniques are limited for use in real time diagnosis with portable devices due to bulky device size and complex measuring processes. | |
| "One of the promising detecting tools for exhaled breath is metal oxide based chemiresistive gas sensors," Il-Doo Kim, an Assistant Professor in the Department of Materials Science and Engineering at Korea Advanced Institute of Science and Technology (KAIST), explains to Nanowerk. "When chemiresistive sensors are exposed to oxidizing or reducing analyte gases, sensor resistivity is changed as a function of temperature as well as of trace gas concentration. The advantage of chemiresistive sensors is that they can offer greater usability for portable real-time breath sensors thanks to their miniaturized size, low cost, easy fabrication, and simplicity of operation." | |
| The most challenging issue in the field of chemiresistive sensors in diagnosis of diseases is the selectivity. In general, exhaled breath consists of tens of VOCs. The oxide materials used in chemiresistive sensors can react with several types of gases. | |
| So far, researchers have focused on finding optimum materials and structural modifications to increase sensor responses. For example, one-dimensional (1-D) oxides, which exhibit a high surface area, have attracted much attention because the mechanisms of chemiresistive sensors are based on a surface reaction between target gas molecules and adsorbed oxygen on oxide (see for instance: "Nanotechnology electronic noses"). The utilization of such 1-D nanostructure materials, however, has shown little progress in applications to gas sensors for diagnosis of diseases because most disease-related gases found in exhaled breath are in very low concentrations. In particular, gas response speed is rather low (> 1 min) and is not suitable for real-time diagnosis. | |
| In new work, reported in the December 13, 2012 online edition of Advanced Functional Materials ("Thin-Wall Assembled SnO2 Fibers Functionalized by Catalytic Pt Nanoparticles and their Superior Exhaled-Breath-Sensing Properties for the Diagnosis of Diabetes"), Kim and his collaborators focused on a method modifying 1-D structures to maximize gas sensor responses. They prove that a chemiresistive sensor can work as a VOCs sensing device to detect very low concentrations of acetone if the sensing materials have optimized morphology and microstructure. | |
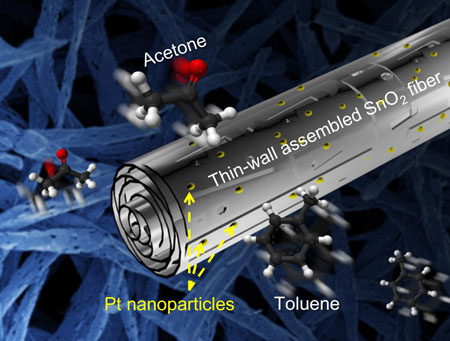 |
|
| Ultrafast acetone sensors using thin-wall assembled SnO2 nanofibers functionalized by catalytic Pt nanoparticles for diagnosis of diabetes. (Image: Dr. Kim, KAIST) | |
| "What we have discovered is the efficacy of porous SnO2 nanofibers consisting of multiple coaxial thin-tubes" says Kim. "This unique morphological feature, with a high surface-to-volume ratio and porous interior structures, can make multiple sensing layers within a single fiber accessible, quickly and effectively." | |
| The team's sensor test results for acetone detectability are around 0.1 ppm, a level which is eight times lower than the required gas-sensing level for diagnosing diabetes, and is the best result among reported SnO2 sensors. | |
| "From our result, we can see a positive prospect for the utilization of this material as an exhaled breath sensor for diagnosing diabetes," adds Kim. "In addition, using the catalytic platinum nanoparticle decoration on the surface of multiple coaxial SnO2 thin-tubes, we can dramatically reduce the gas response time to less than 20 seconds." | |
| The researchers synthesized the thin-walled SnO2 fibers – which are composed of wrinkled SnO2 nanotubes – by electrospinning with controlled phase separation between precursor-rich phases and polymer-rich phases. Electrospinning is a versatile way to produce polymeric, metallic, or metal-oxide fibers; it has been widely studied for application in chemical sensors, bio tissue-engineering, energy storage materials such as Li-ion batteries, electrochemical capacitors, photocatalysts, etc. | |
| When injecting high-dielectric-constant-solutions containing polymers into a sharp needle under a current of tens of thousands of volts, the liquid jet is dragged down to the grounded plate and solidified into polymeric fibers. In particular, researchers have paid attention to an interesting phenomenon called micro phase-separations between polymers and other dissolved solutes. | |
| As Kim explains, the primary feature of micro phase-separation is the possibility to synthesize irregularly shaped fibers such as bundle-type, hollow, and bumpy fibers, which are advantageous for certain applications that require large surface area; this is because the phase separation behaviors, for example, between the Sn precursors and the polymers, can increase the porosity of the SnO2 fibers during heat treatment, during which polymers are burned and left with empty voids between oxidized SnO2 crystals. | |
| "So far, the known factors that induce phase-separation are miscibility differences, solvent properties, humidity, etc." he says. "In addition to these factors, in our work we successfully demonstrate that the variation of flow rate – electrospinning solution feeding rate – can trigger micro phase-separation. The resulting morphology is unprecedented and clearly distinguished from other regular forms of fibers." | |
| Unlike the other forms of phase-separated fibers, this fiber has a unique morphology with a directional property, which the team proves is the result of an increase in the flow-rate. For instance, a high flow-rate intensifies the surface tension and elongation of polymer chains in the longitudinal direction. | |
| Furthermore, the increase in solution supply leads to a higher solvent evaporation rate, which as a whole pushes concentration gradients to the radial direction. Taking two directional phenomena into account, the resulting unique morphology can exhibit elongated open pores along the axis direction and lamellar wall structures along the radial direction. | |
| The researchers believe that these unique characteristics and the mechanisms of the fabrication can be beneficial in various applications that require complex 1-D structures. They also expect that other types of oxide materials can provide superior sensing capacity to react with very low concentrations of VOCs when a similar morphology is properly synthesized. | |
| The priority research objective of Kim's group is to give oxides selectivity to a particular target gas. In a previous paper, they investigated the gas selectivity of WO3, given by the addition of catalytic nanoparticles on the oxide surface ("Exhaled VOCs sensing properties of WO3 nanofibers functionalized by Pt and IrO2 nanoparticles for diagnosis of diabetes and halitosis"). | |
| Decoration of Pt and IrO2 nanoparticles gives WO3 selectivity to acetone and to hydrogen sulfide, which is another type of VOCs. Even though challenges regarding selectivity to acetone in more complex systems with various VOCs remain, we look ahead to a bright future of utilizing exhaled breath chemiresistive sensors to diagnose diabetes. | |
| As part of their effort, the team has developed a prototype of a portable exhaled breath sensor for which, in December 2012, they were awarded the Commissioner of Korean Intellectual Property Office Prize. | |
| "We concede that we still have a long way to go" says Kim. "Through further optimization in the areas of material selection, micro-structuring, and functionalizing with various catalysts, however, we expect in the foreseeable future to make significant progress in the development of exhaled breath sensors with high accuracy and superior selectivity." | |
| Eventually, the final goal is to integrate these novel exhaled sensors into smartphones, allowing users to easily monitor their health anytime, anywhere. This project is already getting a lot of attention from several companies such as Samsung Electronics, Innochips Technology, Amotech, and other mobile phone companies. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
