| Posted: Jul 18, 2013 | |
Silicon chips inserted into living cells can feel the pressure (w/video) |
|
| (Nanowerk Spotlight) The study of individual cells is of great importance in biomedicine. Many biological processes incur inside cells and these processes can differ from cell to cell. The development of micro- and nanoscale tools smaller than cells will help in understanding the cellular machinery at the single cell level. All kinds of mechanical, biochemical, electrochemical and thermal processes could be studied using these devices. | |
| In a previous Nanowerk Spotlight back in 2010, we reported on the work of a research team in Spain to produce silicon chips that can be internalized inside living cells to be used as intracellular sensors ("Future bio-nanotechnology will use computer chips inside living cells"). | |
| In new work, reported in the June 30, 2013, online edition of Nature Nanotechnology ("Silicon chips detect intracellular pressure changes in living cells"), the team, led by José Antonio Plaza, who heads the Micro and Nano Tool group at Instituto de Microelectrónica de Barcelona IMB-CNM (CSIC) and a group from the 3DLab (Development, Differentiation and Degeneration) at Centro de Investigaciones Biológicas in Madrid (CSIC) coordinated by Teresa Suárez, has demonstrated a nanomechanical chip that can be internalized to detect intracellular pressure changes within living cells, enabling an interrogation method based on confocal laser scanning microscopy. | |
| "Our goal was to fabricate a chip small enough to be inserted into a living cell and detect mechanical loads," Rodrigo Gómez-Martínez, the paper's first author, tells Nanowerk. "As a result, we have been able to show that this nanostructured device can detect intracellular pressure changes. This is the first time that pressure can be detected by a sensor located inside an intact cell, preserving the integrity of the cell membrane." | |
| Suárez points out that the presence of the nanochip does not seem to affect the cell's structure or viability – cells with the sensor chip inside were alive, healthy and able to divide (with the sensor remaining inside one of the cells). | |
| Video shows a cell division of a HeLa cell enclosing a chip device. | |
| The challenge for the researchers was to produce a device with an integrated sensor system small enough to fit inside a cell. | |
| The design that they used comprises a mechanical sensor defined by two membranes separated by a vacuum gap, and an optical reference area. The internalized sensors only represented 0.2% of the total volume of a typical HeLa cell. | |
| "The membranes act as parallel reflecting mirrors, constituting a Fabry-Pérot resonator that is partially transparent for some wavelengths," explains Gómez-Martínez. "An external pressure deflects the membranes and changes the gap; which, in turn, modifies the intensity of the reflected light at the centre of the membranes." | |
| He points out that the presence of a sensor inside a vacuole has several inherent advantages: "First, it can give information about how an external pressure is transmitted mechanically to organelles. Second, it prevents the eventual existence of mechanical cross-sensitivity on the devices because of other organelles or cytoskeletal filaments, which can induce small forces and displacements. Third, better-quality confocal laser scanning microscopy images are obtained when the sensors are immersed in a medium with a uniform refractive index." | |
 |
|
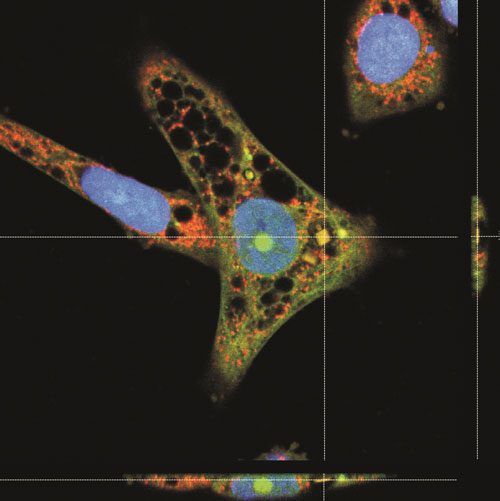
| A HeLa cell displaying an internalized chip. Overlay of confocal images and an orthogonal projection of confocal images showing that the chip is inside the cell. The cells were loaded with vital dyes CellTracker Green and MitoTracker Red before fixation. (Image: Alberto Hernández-Pinto, Centro de Investigaciones Biológicas, CIB (CSIC)) | |
| Extracellular pressure is a common load in many situations. For instance, human cells experience a pressure of 0.2 bar from throughout the body, which can increase during certain activities; and deep-sea animals can be exposed to 200 bar upon diving. | |
| "Our experiments support the supposition that the cytoskeletons of human HeLa cells do not mechanically withstand extracellular pressures in the studied range and under our experimental cell culture conditions," says Suárez. "Thus, extracellular pressure is transmitted through the cytosol to the inner compartments. The implication is that intracellular transmission of fluid pressure follows Pascal's law." | |
| Going forward, the team will try to improve their sensor by increasing its sensitivity and include thinner mechanical layers, autofocus and tilt-stage systems, and computer-assisted measurements. | |
| Another objective is to design chips that can measure other intracellular parameters – biochemical, electrical, thermal etc. – in order to investigate more complex cellular processes. | |
| "Intracellular mechanical sensors will provide information directly from inside the cellular environment about these cellular forces and will provide new opportunities," notes Plaza. "We believe that this is a first step towards a wide-ranging field of intracellular nanochips that will offer a different perspective on fundamental problems in cell biology." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
