| Posted: Oct 28, 2013 | |
Automated and scalable inline two-stage synthesis process for high quality colloidal quantum dots |
|
| (Nanowerk Spotlight) Colloidal quantum dot (CQD) nanocrystals are attractive materials for optoelectronics, sensing devices and third generation photovoltaics, due to their low cost, tunable bandgap – i.e. their optical absorption can be controlled by changing the size of the CQD nanocrystal – and solution processability. This makes them attractive candidate materials for quantum dot displays and cheap and scalable roll-to-roll printable device fabrication technologies. | |
| One key impediment that currently prevents CQDs from fulfilling their tremendous promise is that all reports of high efficiency devices were from CQDs synthesized using manual batch synthesis methods (in classical reaction flasks). Researchers have known that chemically producing nanocrystals of controlled and narrow size-distributions requires stringent control over the reaction conditions – e.g. temperature and reactant concentration – which is only practical for small-scale reactions. Such a synthesis is extremely difficult to scale up, hence very costly to mass produce without severely compromising quality. | |
| The reason for this is that, just like rain droplets, nanocrystals form sequentially by 'nucleation' and 'growth'. Both these phenomena are highly sensitive to temperature and reagent concentration. Moreover, nucleation and growth must occur at substantially different temperatures and, in fact, to obtain nanocrystals of uniform sizes, one must be able to rapidly cool down the reaction from the nucleation temperature to the growth temperature. Hence, the quality of the product is contingent upon how well and fast one can homogenize the reactor, both chemically and thermally. | |
| Unfortunately, the only way to scale up batch reactors is by increasing their volume, whereupon it becomes difficult to homogenize the reactor and impractical to rapidly cool. The end result is nanocrystals of low-quality and broad size distributions, which are not useful for fabricating devices. | |
| Some researchers have sought to circumvent this limitation by conducting the reactions in narrow fluidic channels (less than a 1 mm in diameter) while the reactants are continuously pumped through the channels, so called 'continuous-flow reactors'. | |
| Conceptually, this scheme has several advantages. Narrow-width channels afford uniform heating and mixing of the reaction, while the reaction is scalable by simply increasing channel length and pump rate of the reagents. This sort of scaling does not effect the quality of the product, because the channel width, and hence the effective reaction volume, remains the same. Despite these advantages, most attempts to use continuous-flow reactors in the past have resulted in nanocrystals with a much lower quality than the batch produced ones. | |
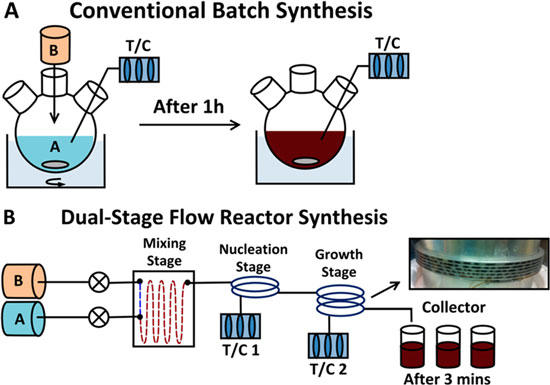
| "We have analyzed the nucleation and growth of CQDs in continuous-flow reactors and realized that, in order to achieve controllable size and narrow size-distributions, one must employ two temperature stages in the reactor: one for nucleation, and another for growth," Osman Bakr, an assistant professor in the Solar & Photovoltaics Engineering Research Center at King Abdullah University of Science and Technology (KAUST), tells Nanowerk. "By separating these two crucial steps in the formation of the CQDs in time, temperature, and space, we were able to obtain very high quality nanocrystals, as good as the best batch synthesis, by a process that is low-cost, mass-producible, and automated." | |
 |
|
| Schematic of (a) a conventional batch synthesis setup and (b) a dual-stage continuous flow reactor setup with precursor A (Pb-oleate, octadecene) and precursor B (bis(trimethylsilyl) sulfide in octadecene). (Reprinted with permission from American Chemical Society) | |
| Reporting their findings in ACS Nano ("Automated Synthesis of Photovoltaic-Quality Colloidal Quantum Dots Using Separate Nucleation and Growth Stages"), Bakr and his team demonstrated the quality of the CQDs produced by their method by using them to make CQD-based solar cells that showed very high efficiencies. | |
| "In this paper, we developed an automated, scalable, in-line synthesis methodology of high-quality CQDs based on a flow-reactor with two temperature-stages of narrow channel coils," says Professor Ted Sargent from the University of Toronto who, together with Bakr, led this work. "The flow-reactor methodology not only enables easy scalability and cheap production, but also affords rapid screening of parameters, automation, and low reagent consumption during optimization. Moreover, the CQDs are as good in quality and device performance as the best CQDs that are produced in the traditional batch methodology." | |
| The team also developed a general theory for how one can use the flow-reactors to finely tune the quality and size distribution of the CQDs, and explained why previous attempts of using flow-reactors based on a single-temperature-stage, as opposed to a dual-temperature-stage, necessarily produce CQDs of low-quality and broad size distribution. | |
| This work paves the way towards the large-scale and affordable synthesis of high-quality CQD nanocrystals in tunable sizes, enabling photovoltaics, light-emitting diodes, photodetectors, and biological tagging technologies that take advantage of the nanoscale properties of those promising materials. | |
| "Over the last ten years we have seen tremendous advancements in software and computer integration, in items that we use in our everyday lives," says Bakr. "Flow-reactors as a platform are ideally placed to take advantage of this trend. Software that automates the routines of flow-reactors already exists. In the near future, researchers will be able to run and monitor hundreds of experiments to produce CQDs from home using a mobile app. Moreover, because flow-reactors contain very few moving parts, essentially just programmable pumps, I expect that it will become an automated research platform that most labs studying nanocrystals can afford." | |
| "Our work has shown that flow-reactors can produce nanocrystals that are as good as the best batch produced reactions, with exquisite control over reaction conditions," he adds. "We believe that this will encourage the nanomaterials community to take advantage of the enormous productivity gains in R&D afforded by flow-reactors, which other chemical industries, such as pharmaceuticals, are currently utilizing earnestly." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
