| Posted: Dec 03, 2007 | |
Nanotechnology candy to thwart bioterrorism and food contamination |
|
| (Nanowerk Spotlight) Talking about the threat of terrorists using bioweapons is a great tool for scaring people. Using any kind of pathogen (bacterium, virus or other disease-causing organism) as a weapon certainly is a terrifying scenario; think about the near-panic the 2001 anthrax attacks in the United States caused. Letters containing anthrax spores were mailed to several news media offices and two U.S. Senators, killing five people and infecting 17 others. Can you image what panic would result from an attack that kills 5,000 people and causes 76 million illnesses? Well, as a matter of fact, foodborne diseases cause approximately 76 million illnesses, 325,000 hospitalizations, and 5,000 deaths in the United States each year. | |
| Known pathogens account for an estimated 14 million illnesses, 60,000 hospitalizations, and 1,800 deaths CDC data). The Food and Drug Administration’s (FDA’s) 2005 Food Code states that the estimated cost of foodborne illness is $10–$83 billion annually. So while the U.S. spends billions of dollars securing its borders, it loses many more billions, not to mention thousands of lives, every year by not being able to keep its spinach and hamburgers safe. Apparently, talking about terrorism is much better political theater (and makes for catchier Nanowerk Spotlight titles) than discussing E. coli outbreaks. However, be it because of potential terrorists or actual contaminated food, research in microbial detection and decontamination processes increased significantly over the past years. Traditional methods of identifying and subsequently removing a pathogen are slow and cumbersome. Now, using nanotechnology, researchers have designed a novel biosensing system that can identify E. coli in just five minutes and remove up to 88% of the target bacteria. | |
| Traditionally, identifying a pathogen such as E. coli, Salmonella or Listeria requires cell culturing, which takes time – time that often means more contamination and illnesses or even deaths. Here is an example from the FDA's recommended method for determining E. coli: | |
| Weigh 50 g food into sterile high-speed blender jar. Add 450 mL of Butterfield's phosphate-buffered water and blend for 2 min. Prepare decimal dilutions with sterile Butterfield's phosphate diluent. Number of dilutions to be prepared depends on anticipated coliform density. Shake all suspensions 25 times in 30 cm arc or vortex mix for 7 s. Do not use pipets to deliver <10% of their total volume. Transfer 1 mL portions to 3 LST tubes for each dilution for at least 3 consecutive dilutions. Hold pipet at angle so that its lower edge rests against the tube. Let pipet drain 2-3 s. Not more than 15 min should elapse from time the sample is blended until all dilutions are inoculated in appropriate media. Incubate LST tubes at 35°C. Examine tubes and record reactions at 24 ± 2 h for gas, i.e., displacement of medium in fermentation vial or effervescence when tubes are gently agitated. Re-incubate gas-negative tubes for an additional 24 h and examine and record reactions again at 48 ± 2 h. Perform confirmed test on all presumptive positive tubes (which takes another 2 days). | |
| It is a nobrainer that a detection system that takes days to positively identify a potentially deadly pathogen contamination is not good enough. What is urgently needed is a rapid way to detect the presence of a pathogen as well as the strain identity. That's were nanotechnology techniques could come to the rescue. | |
| "We demonstrate the potential of sugar-coated magnetic nanoparticles for fast bacterial detection and removal, which provides an attractive avenue for pathogen decontamination and diagnostic applications" Dr. Xuefei Huang tells Nanowerk. | |
| Huang, an Associate Professor in the Department of Chemistry at the University of Toledo, together with his collaborators from the university, developed a magnetic glyco-nanoparticle (MGNP)-based system to not only detect E. coli within 5 minutes, but also to remove up to 88% of the target bacteria from the medium. This system also allows easy determination of the identities of three different E. coli strains on the basis of the response patterns to two MGNPs highlighting their potential in biosensing. | |
| The findings have been reported in a recent article in the Journal of the American Chemical Society ("Magnetic Glyco-nanoparticles: A Unique Tool for Rapid Pathogen Detection, Decontamination, and Strain Differentiation"). | |
| Huang and his team decided to use magnetic nanoparticles since their high surface/volume ratio offers more contact surface area for attaching carbohydrates and for capturing pathogens. Nanoparticles typically are about two orders of magnitude smaller than a bacterium, allowing many nanoparticles to attach to a bacterial cell, which aids in removing the bacteria. | |
| "Pathogens such as bacteria and viruses often have a 'sweet tooth' which allows them to bind with mammalian cell surface carbohydrates to initiate infection" Huang explains. "To mimic this effect, we decorated the surface of MGNPs with carbohydrate moieties capable of binding surface recognition elements. This leads to particles with robust recognition capabilities and with the advantage of being magnetic." | |
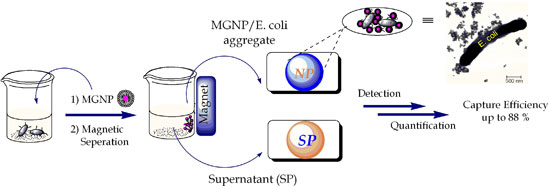 |
|
| Schematic demonstration of pathogen detection by MGNPs. (Image: Dr. Huang, University of Toledo) | |
| In their experiments, Huang and his team found that when the MGNP are introduced to a pathogen, the pathogen latches onto the sugar. Once the pathogen has been captured, using a magnetic field the researchers were able to remove up to 88% of the E. coli, 65% in the first five minutes. This is much higher than most current systems using antibodies. | |
| In addition, the technique can differentiate one pathogen strain from another. Because various strains of bacteria can have very different binding affinities with a sugar, the response patterns of bacteria to MGNPs can be deciphered allowing the identification of the bacterium strain. | |
| Huang points out that the principle of this is very similar to how our tongues detect different flavors or how our noses register different smells. "Our technique is unique as it can be used to detect, decontaminate, and differentiate bacteria using the same system" he says. | |
| It appears that this technique has considerable commercial potential: The magnetic glyco-nanoparticles are very simple and inexpensive to fabricate. The carbohydrate moieties on the exterior surface can be easily varied to adapt for specific pathogens. Unlike antibodies, they are also very stable at room temperature and thus do not need special facilities to store, which bodes well for field application. | |
| The possibility of using this system not only to rapidly detect but also to decontaminate a suspected sample makes it particularly attractive. | |
| "Our proof-of-principle demonstration – using a MGNP system to rapidly identify and decontaminate E. coli – not only paves the way for developing nanoparticles with more complex carbohydrates but also exhibits the power of nanotechnology in biosensing" says Huang. "In the next stage of work, we will use glyco-nanoparticles to differentiate a larger number of different pathogens. We will work on enhancement of detection limit and specificity. Besides pathogens, we also plan to examine the possibility of detecting cancer cells using this technique." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
