| Posted: Aug 13, 2014 | |
A convenient method to chemically modify boron nitride nanotubes |
|
| (Nanowerk Spotlight) First theorized in 1994 by researchers at UC Berkley ("Theory of graphitic boron nitride nanotubes"), boron nitride nanotubes (BNNT) represents a new class of super-strong materials. These textile-like nanotubes with the appearance of cotton have a molecular backbone 100 times stronger than steel. They are as strong as the better-known carbon nanotubes (CNTs), but much more heat resistant – up to 800°C in air and very resistant to many chemical modifications. | |
| "So far, it has been generally accepted knowledge that BNNT are highly inert to oxidative treatments and can only be covalently modified by highly reactive species," Zhongfang Chen, a Professor in the Department of Chemistry at the University of Puerto Rico (UPR), tells Nanowerk. "By contrast, oxidation of CNTs has been proven very convenient and fundamentally important to modify the nanotube structure and morphology via controlled corrosive effects." | |
| In new work, reported in Advanced Functional Materials ("Chemical Sharpening, Shortening, and Unzipping of Boron Nitride Nanotubes"), Chen and his collaborators, a group of researchers led by Dr. Yi Lin (National Institute of Aerospace, NIA) and Dr. John W. Connell (NASA Langley Research Center), have discovered a convenient method to disperse and chemically modify the morphology of BNNTs by sonication in aqueous ammonia solutions. | |
| These findings overthrow the general beliefs about the high chemical stability of boron nitride nanotubes. | |
 |
|
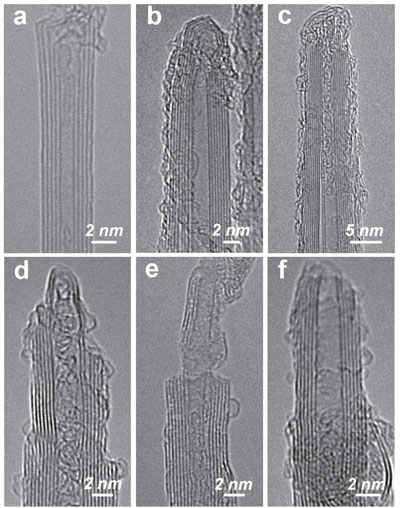
| HR-TEM images of (a–c) pristine BNNTs, and also various modified BNNTs from sonication in (d–f) 3% aqueous ammonia solution for 2 h, (g–i) 3% for 8 h, and (j–l) 10% for 2 h. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| "We found that BNNTs can be chemically dispersed, and their morphology can be modified by a relatively mild method: simply sonicating the nanotubes in aqueous ammonia solution at ambient conditions," notes Chen. "It is not surprising that ammonia, as a small Lewis base molecule, can be used for such a purpose since similar chemistry is well known. However, unexpectedly but more importantly, the nanotube structures were significantly corroded." | |
| As the team reports in their paper, the observed corrosive effects included various morphologies such as nanotube end-cap removal, tip sharpening, sidewall thinning, length shortening, and also partial or even full longitudinal unzipping. | |
| As Chen explains, the team's detailed TEM investigations on the ammonia-modified BNNTs revealed significant corrosion of these presumably inert nanotubes. Compared to the pristine BNNTs with highly crystalline sidewalls and intact end-caps (a-c in the figure above), the ammonia-modified BNNTs, even with only 2 hours sonication using 3% aqueous ammonia solution, showed significant wall corrosion and thinning near the nanotube tip, an effect that might be referred to as 'sharpening' (d,e in figure above). The end-caps of some nanotubes were completely removed so that the tubes became open-ended. | |
| "While BNNTs are inert to strong oxidative treatment conditions, their structural vulnerability to aqueous ammonia solutions opens up a pathway which may allow their convenient processing toward a variety of applications," says Chen. | |
| This work follows Lin and Connell's previous work using sonication-assisted hydrolysis to disperse few-layer and monolayer boron nitride nanosheets in water ("Aqueous Dispersions of Few-Layered and Monolayered Hexagonal Boron Nitride Nanosheets from Sonication-Assisted Hydrolysis: Critical Role of Water"). “What we found then was that water molecules can attack and dissemble boron-nitride network under continuous sonication,” Lin said, “what we found now is that, with the presence of ammonia, similar network in boron nitride nanotubes becomes much weakened and more vulnerable to such attack.” Lin explained that, as a Lewis base, electron-rich ammonia molecules in a large amount can effectively attach onto the electron-deficient boron atoms on the nanotubes, causing the weakening of the boron-nitride network. | |
| This study may pave the way for convenient processing of BNNTs, previously thought to be highly inert, toward controlling their dispersion, purity, lengths, and electronic properties. | |
| In particular, this is the first chemical method to obtain boron nanoribbons from BNNT unzipping. | |
| The structural vulnerability of BNNTs to such simple chemical treatments opens up a pathway which allows their more convenient processing for various applications, such as BN-based nanosensors and nanocapacitors. | |
| Among others, this work offers simple procedures for sorting and controlling the BNNT length, purification of BNNTs, and production of BN nanoribbons as a novel type of high aspect ratio two-dimensional material. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
