| Posted: Jan 04, 2016 | |
Identifying nanopollution |
|
| (Nanowerk Spotlight) Engineered nanoparticles are being used in a wide range of product areas, including composite materials, coatings, electronics, food, agriculture, cosmetics, healthcare, and biotechnology. As a consequence, human exposure to nanoparticles has become a prominent environmental concern; especially since these potential pollutants are not visible to the human eye or detectable by smell. However, there is no current technology that provides rapid, sensitive and highly portable detection and identification of nanoparticles. | |
| Now though, researchers have developed a simple colorimetric sensor array approach capable of detection and unambiguous differentiation of a wide range of nanoparticles in aqueous solutions. | |
| "We have demonstrated a colorimetric sensor array capable of detection and unambiguous differentiation of a wide range of nanoparticles i.e., gold nanospheres, gold nanorods, and multifunctional carbon nanospheres," Morteza Mahmoudi, an assistant professor at Tehran University of Medical Sciences, who heads the Laboratory of Nano-Bio Interactions, tells Nanowerk. "Our sensor array is able to discriminate among different sizes, shapes, and compositions of nanoparticles over a range of concentrations. This approach makes it possible to monitor the existence of nanopollution in the environment, such as water resources." | |
| Mahmoudi worked with chemistry professor Kenneth Suslick and his group at the University of Illinois at Urbana-Champaign, and the team reported their findings in ACS Sensors ("Identification of Nanoparticles with a Colorimetric Sensor Array"). | |
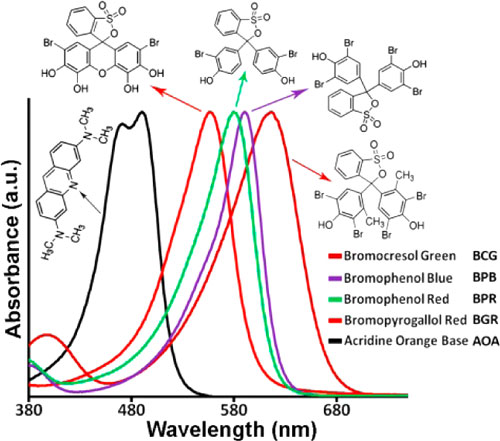 |
|
| Structures, absorbance spectra, and abbreviations of the five dyes used in the colorimetric sensor array at pH of 7.4. (Reprinted with permission by American Chemical Society) | |
| "We found the interactions of a wide range of nanoparticles with cross-reactive chemoresponsive dyes, whose visible absorbances change in response to their interactions, provide a unique color change profiles for each type of nanoparticles," Mahmoudi and Suslick explain. "The interactions between dyes and nanoparticles could be a summation of local pH effects, Lewis acid-base interactions, hydrogen bonding, and local polarity (solvatochromic effects). To that end, the nature of the interfacial region of nanoparticles is a reflection of the physicochemical properties of each type of nanoparticle and therefore provides a means of nanoparticle identification." | |
| To develop their assays, the researchers employed five water-soluble chemoresponsive dyes – specifically bromocresol green (BCG), bromophenol blue (BPB), bromophenol red (BPR), bromopyrogallol red (BGR), and acridine orange base (APB). | |
| This colorimetric approach may pave the way for a fast, reliable, and inexpensive method to detect the presence of nanoparticles – in a worst case, 'nanopollution' – and to characterize the physiochemical properties of nanoparticles at very low concentration. | |
| "The colorimetric sensor array approach has generally used printed dye arrays on porous membranes," Mahmoudi points out. "For application to the identification of nanoparticles, however, we made use of an array of solution phase sensors and generated color difference maps using changes in the visible absorbance spectra of the dyes in aqueous solutions before and after exposure to the nanoparticles." | |
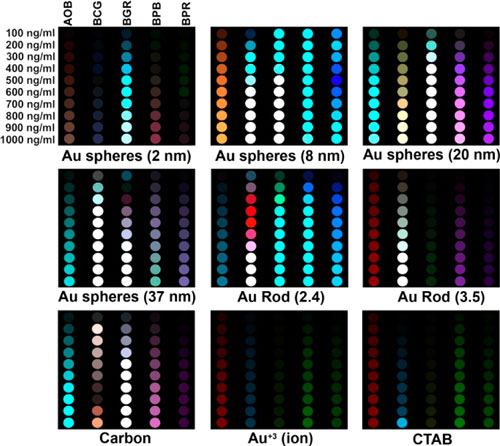 |
|
| Color-change profiles of the five sensor dyes after interaction with various nanoparticles at different nanoparticle concentrations. For display purposes, these difference maps were generated by subtraction of the solution absorbance before exposure from that after exposure to various nanoparticles and controls (i.e., gold ions (Au3+) and surfactant (CTAB)) with three selected wavelengths (i.e., 480, 590, and 620 nm) assigned to RGB values; at each of these three wavelengths, absorbances from 0 to 0.484 optical density were mapped linearly to 0 to 255 in RGB values. (Reprinted with permission by American Chemical Society) | |
| In order to ensure reliable measurements of the color differences of the dyes before and after interaction with nanoparticles, the scientists performed all measurements under tight control of pH at 7.41 using standard phosphate buffered saline solutions. | |
| Mahmoudi notes that, besides environmental applications, he expects these results to find application in the emerging field of nanomedicine. "Colorimetric approaches may be used for the detection and identification of various protein corona coated nanoparticles," he says. | |
| In a previous Nanowerk Spotlight ("Personalized protein coronas result in different therapeutic or toxic impacts of identical nanoparticles") we reported how the identification of protein corona coated nanoparticles will find applications in the detection of various severe diseases like cancer. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
