| Posted: Feb 05, 2016 | |
Real-time and label-free detection of single viruses in complex solutions |
|
| (Nanowerk Spotlight) Every outbreak of a deadly or dangerous virus somewhere in the world – like last year the Ebola virus in West Africa, or just now the Zika virus in Latin America – highlights the importance of rapid detection and accurate diagnosis in health care and preventative medicine. Early detection of these diseases is a critical tool in helping control the virus and save lives. | |
| The problem is that many virus detection platforms, including conventional fluorescent label-based ones, have limitations because they are time-intensive and not easily compatible with point-of-care use without the existence of significant infrastructure and expert staff. Case in point is electron microscopy, frequently used for the direct imaging of viruses and nanoparticles, but not suitable for field operations. | |
| "Various novel optical sensor systems have been developed in an attempt to meet the needs of point-of-care environments," M. Selim Ünlü, Distinguished Professor of Engineering in the Optical Characterization and Nanophotonics Laboratory (OCN Lab) at Boston University, tells Nanowerk. "Each of these optical sensors has unique benefits, but none has truly filled the gap to provide robust, direct, and chemically specific high-throughput single-particle visualization in a complex sample. There remains a need for a bridging technology between traditional wide-field microscopy and high-resolution electron microscopy." | |
| In new work, Ünlü's group and their collaborators have mated an interferometric reflectance imaging sensor developed in their lab with a microfluidic cartridge that allows them to dynamically image the sensor and visualize very small nanoparticles in solution. | |
| The researchers reported their findings in the January 13, 2016 online edition of ACS Nano ("Real-Time Capture and Visualization of Individual Viruses in Complex Media"). | |
| This is the first technique capable of specifically visualizing label-free single viruses in complex solutions in real-time. | |
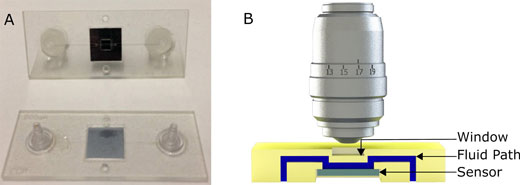 |
|
| (A) Picture of the first generation polymeric cartridge used for virus and nanoparticle imaging. (B) Cross section model which demonstrates the fluidic path (in blue), the sensor (in gray) and the chip and window (in yellow) not to scale. (Reprinted with permission by American Chemical Society) | |
| "We developed a simple to use tool that extends the range of light microscopy to smaller size particles allowing visualization of viruses and nanoparticles that used to require specialized liquid electron microscopy techniques," notes Steven M. Scherr, a Graduate Research Assistant in the OCN Lab and first author of the paper. | |
| The OCN team and its collaborators have spent the past several years advancing a rapid, label-free, chip-scale photonic device that can provide affordable, simple, and accurate on-site detection. They first demonstrated the concept back in 2010 (Nano Letters, "High-Throughput Detection and Sizing of Individual Low-Index Nanoparticles and Viruses for Pathogen Identification"). In 2014, a team led by Ünlü and MED associate professor of microbiology John H. Connor followed this up with a study where they showed the capture and imaging of Ebola and Marburg surrogates using this technology (ACS Nano, "Digital sensing and sizing of vesicular stomatitis virus pseudotypes in complex media: a model for Ebola and Marburg detection"). | |
| Now, with this new work, they have refined their instrumentation to allow the simultaneous detection of multiple viruses in blood serum samples—including viruses genetically modified to mimic the behavior of Ebola and the Marburg virus. | |
| "With our sensor we can see thousands of individual viruses or nanoparticles in real-time while they are still in a natural environment, such as serum or plasma," says Scherr. "This lets us take dynamic measurements and visually investigate a large number of viruses in solution in a way that was previously not possible." | |
| "Furthermore" he adds, "as a detection system, our approach eliminates virtually all sample preparation. Since the viral pathogens are directly (without labels) visualized, detected, and discriminated from other potentially interfering noise in the target solution, no wash step is needed." | |
| The team has named their prototype diagnostic device the Single-Particle Interferometric Imaging Sensor (SP-IRIS). It allows for the study of unmodified viruses using visible light microscopy. It works by shining light from multi-color LED sources on viruses that are bound to the sensor surface by a coating of virus-specific antibodies. Interference of light reflected from the surface is modified by the presence of the viruses, producing a distinct signal that reveals the size and shape of each virus. | |
| The scientists point out that this technique can help bridge the gap between light microscopy and electron microscopy and be applied to both research and diagnostic applications. This tool will let researchers explore populations of synthetic and bio-nanoparticles (viruses, cellular vesicles, etc.) in solution. The sensor can also be used as a rapid and sensitive diagnostic tool. | |
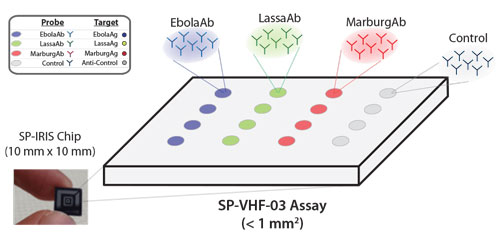 |
|
| In a multiplexed detection configuration, Ebola, Marburg and Lassa antibodies on the SP-IRIS surface capture corresponding viruses specifically. As shown in the images produced by the device, SP-IRIS then identifies and counts each virus particle and classifies it based on its size. (Image: NexGen Arrays) (click on image to enlarge) | |
| "We are continuing to develop this as a rapid viral hemorrhagic fever test," says Scherr. "This technique lets us test for Ebola as well as several other similar diseases in a single test that is both fast and sensitive and requires less sample preparation than other highly sensitive techniques." | |
| The team's collaborators at The University of Texas Medical Branch at Galveston (Geisbert Lab) will begin testing this cartridge with bona fide Ebola samples in the near future. The experiments will be done using the in-liquid imaging approach described in the manuscript using an automated reader created by Nexgen Arrays, a spinout company that commercializes the SP-IRIS platform. | |
| "We would like to see a highly multiplexed virus detection panel that can differentiate between a number of viruses that present with similar symptoms available in an easy-to-use disposable cartridge platform," adds Ünlü. "Also, our technique allows for characterization of viruses. An exciting new application area for our technology is the quality control application for the emerging oncolytic viruses." | |
| Video: This movie demonstrates virus binding and single virus detection. It represents the actual binding sped up by a factor of 100. The initial images at the beginning of the experiment show very few particles present on the sensor surface. Accumulation of virus begins rapidly and is sustained for the 60 minute experiment. The movie was created using the images acquired from the SP-IRIS sensor system with minimal postprocessing (AVI). (Video: Optical Characterization and Nanophotonics Laboratory at Boston University) | |
| The team wants to continue to push the limit on the smallest particles they can see with this technique and improve the cartridge to make the test easy enough for anyone to use. Although imaging ultra-small particles in solution is difficult, optical detection remains an invaluable tool. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
