| Posted: Oct 05, 2016 | |
Nanotube chip captures and analyzes circulating tumor cells in blood |
|
| (Nanowerk Spotlight) Early and accurate detection of cancer is critical for successful cancer therapies. In most cases, a tissue biopsy is the initial means of making a diagnosis. With increasing accuracy, liquid biopsies – where circulating tumor cells (CTCs) are isolated from blood samples – are becoming a viable complement or even alternative to invasive biopsies of metastatic tumors. | |
| CTC is of great interest for evaluating cancer dissemination, predicting patient prognosis, and also for the evaluation of therapeutic treatments, representing a reliable potential alternative to invasive biopsies and subsequent proteomic and functional genetic analysis. | |
| Unfortunately, given that human blood is a complex fluid that contains a variety of cells and metabolites, the fast detection of CTCs is quite a difficult task. | |
| For the past 15-20 years, benefitting from improving microfluidic techniques and other nanotechnology in healthcare developments, scientists have been getting better and better at isolating CTCs. | |
| Most CTC isolation techniques are based on EpCAM (a protein known as epithelial-cell adhesion molecule; EpCAM is expressed on the surface of a wide variety of solid-tumor cells). | |
| "EpCAM based CTC isolation has limitations as EpCAM is often down-regulated during metastasis; further, most CTC isolation these days is based on some form of microfluidics," Balaji Panchapakesan, an Associate Professor in the Department of Mechanical Engineering at Worcester Polytechnic Institute, tells Nanowerk. "Our work shows a brand-new philosophy in capturing CTCs that is not immunomagnetic and microfluidic enrichment based." | |
| Reporting their work in the September 29, 2016 online edition of Nanotechnology("Static micro-array isolation, dynamic time series classification, capture and enumeration of spiked breast cancer cells in blood: the nanotube–CTC chip"), Panchapakesan and his collaborators demonstrate a completely new micro-array design that is looking at capture and detection of CTCs from an entirely new perspective. | |
 |
|
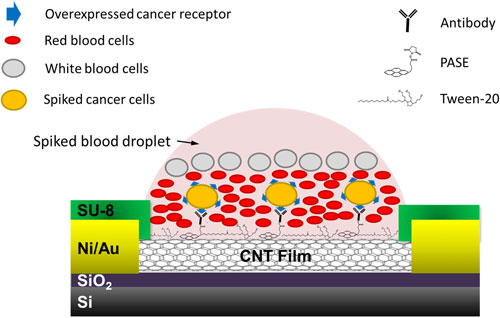
| Schematic of the device for capture of cells spiked in blood. (© IOP Publishing) (click on image to enlarge) | |
| Rather than using magnetic and microfluidic methods that are used in isolating CTCs, the team used a static isolation in the form of micro-arrays of single-walled carbon nanotubes (SWCNTs). Using photo-lithography, metal deposition, and etching techniques, they fabricated chips containing a 76-element array of SWCNT devices that are functionalized with anti-EpCAM, anti-human epithelial growth factor receptor 2 (anti-Her2) and non-specific immunoglobin (IgG) antibodies. | |
| "Our nanotube-CTC chip incorporates both detection and capture technology," Panchapakesan points out. "It is also a self-enrichment and leukocyte depletion process the way blood settles on the device. This is due to the hydrophobic nature of nanotubes resulting in droplet localization and size and density based settling of cancer cells, red blood cells and leukocytes." | |
| He adds that this is a detection and capture technology that captures large numbers of viable spiked breast cancer cells in whole blood without pre-labeling, pre-fixation, or any other processing steps. "Blood can simply be adsorbed and electrical sensing and dynamic time warping (DTW) classification can enable detection and stratification. Classified devices can then be analyzed using optical/confocal microscopy on chip." | |
| The nanotube–CTC chip can be customized to different cancers, conduct combinatorial studies on cancer cells and potentially can save time and provide more information about the disease. | |
 |
|
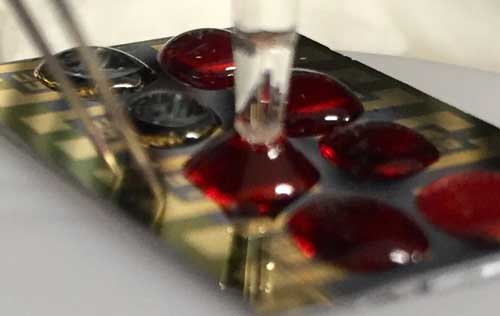
| Test set-up showing the blood droplet, source, drain and reference electrode. (Image: Balaji Panchapakesan) | |
| The team's results suggest that the micro-array format can give rise to similar yields as microfluidic enrichment. CTC loss is avoided as there are no shear forces unlike microfluidics where the CTC capture yield depends on the flow rate. The higher the flow rate, the smaller the yield making all microfluidics slow in CTC enrichment. | |
| Furthermore, since this is a static micro-array isolation, it becomes possible to capture more elusive 30 nanometer structures called exosomes, which are secreted by cancer cells and have the same surface marker profile. Microfluidics cannot capture exosomes as they will be washed away/destroyed due to shear forces. | |
| The chip can also isolate cells based on their biomarker profiles. Multiple biomarkers such as EGFR and Her2 are indicative of metastatic disease and therefore, this technique could be used to make multiple biomarker based captures. | |
| The next step for the researchers is to test their nanotube–CTC chips in patients with different stages of cancer and validate this technology further to enable capture of rare CTCs in a single blood test using different markers. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
