| Posted: Feb 15, 2008 | |
Break a bone - and fix it with carbon nanotubes |
|
| (Nanowerk Spotlight) Carbon nanotubes (CNTs) have shown promise as an important new class of multifunctional building blocks and innovative tools in a large variety of nanotechnology applications, ranging from nanocomposite materials through nanoelectronics to biomedical applications. The exploration of CNTs in biomedical applications is well underway and exploratory uses have included CNT-coated implants, drug delivery and CNTs as components of biosensors. Notwithstanding the still not satisfactorily addressed issue of toxicity, CNTs' properties such as high strength, high electrical and thermal conductivities, and high specific surface area render them particularly useful in the fabrication of nanocomposite-derived biomedical devices. | |
| In one particular area - biomaterials applied to bone - CNTs are anticipated to improve the overall mechanical properties for applications such as high-strength arthroplasty prostheses expected to remain in the body for a long time, or fixation plates and screws that will not fail or impede healing of bone. In addition, CNTs are expected to be of use as local drug delivery systems or scaffolds to promote and guide bone tissue regeneration. A new study by Japanese scientists clearly demonstrates that multi-walled CNTs (MWCNTs) have good bone-tissue compatibility, permitting bone repair and becoming closely integrated with bone tissue. Furthermore, under certain circumstances, their results indicate that MWCNTs accelerate bone formation. | |
| "Bone-tissue compatibility is extremely important for using CNTs in biomaterials placed in contact with bone, but no studies have characterized this property or another very important one: the effects of CNTs on bone healing and bone regeneration" Dr. Naoto Saito explains to Nanowerk. "Our recent study is the first to clarify the bone-tissue compatibility of CNTs and their influence on new bone formation to determine whether and how CNTs might perform in biomaterials in contact with bone or as scaffolding for bone regeneration." | |
| Saito, a professor in the Department of Applied Physical Therapy at Shinshu University School of Health Sciences in Matsumoto, Japan, together with colleagues from the university's School of Medicine, and Faculty of Engineering, published the study's results in the January 18, 2008 online edition of Small ("Carbon Nanotubes with High Bone-Tissue Compatibility and Bone-Formation Acceleration Effects"). | |
| "Our study clearly demonstrated that multi-walled carbon nanotubes have good bone tissue compatibility, permitting bone repair and becoming closely integrated with bone tissue" says Saito. "Furthermore, our results indicate that MWCNTs accelerate bone formation in response to bone morphogenetic protein." | |
 |
|
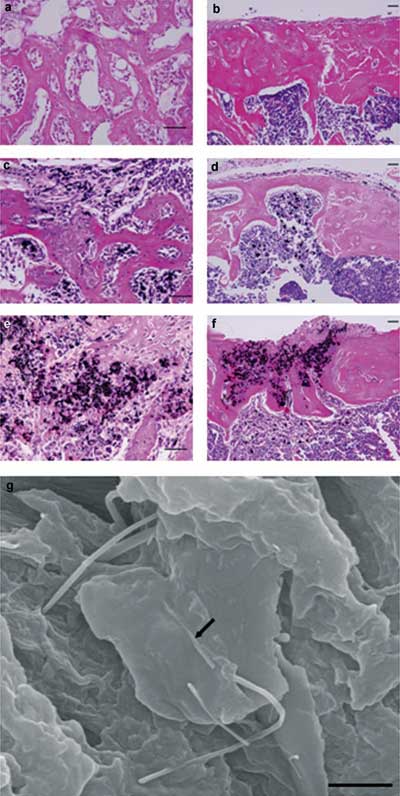
| MWCNTs do not inhibit bone repair and are integrated into new bone through direct contact. Histologic appearance of the tibial defects in mice 1 week and 4 weeks, respectively, after operating: a,b) the sham operation group receiving no implant, c,d) the control group with graphite, and e,f) the MWCNT group. a) Active bone formation was seen at the bone defect and bone repair progressed. b) Bone-tissue repair was complete. Cortical bone was fully formed, and the bone marrow cavity was restored. c) New bone formation was insufficient and bone repair was delayed. Graphite particles were not seen in the new bone matrix. d) Bone repair was clearly inhibited, and newly formed cortical bone and trabecular bone were thin. Graphite particles were seen only in bone marrow tissue, not in bone matrix. e) Bone formation in the defect progressed to the same degree as in the sham operation group. MWCNT particles were incorporated into newly formed bone matrix. f) Complete repair of the bone defect was seen. Cortical bone was formed, and the bone marrow cavity was repaired. MWCNT particles were incorporated not only into bone marrow tissue but also into bone matrix. g) Field emission scanning electron microscopy (FE-SEM) image of tissue sections obtained 4 weeks after operation. MWCNTs (arrow) were integrated directly into the new bone and adhered closely to the bone matrix with no intervening space. Haematoxylin and eosin staining for (a–f). Scale bars: a–f) 100 µm, g) 1 µm. (Reprinted with permission from Wiley-VCH Verlag) | |
| In their study, the Japanese scientists tested highly crystalline MWCNTs with an average diameter of 80 nm and a length from 10 to 20 µm, prepared by catalytic chemical vapor deposition and subsequent thermal treatment above 2800°C in argon. They note that the high-temperature treatment was a critical step to improve the structural integrity of the their high-purity MWCNTs (ca. 98%) and also to remove any remaining metallic impurities below 100 ppm. | |
| Saito and his collaborators basically tested four issues: 1) Are the MWCNTs compatible with bone tissue? 2) What is their influence on bone healing? 3) What is their influence on new bone formation? and 4) Do MWCNTs promote hydroxyapatite crystallization (70 percent of bone is made up of this inorganic mineral)? | |
| With regard to compatibility, the experiments indicated that MWCNTs did not cause strong inflammatory reactions and had no influence on bone even when in contact with it, representing good bone-tissue compatibility. | |
| To examine their influence on bone healing, the researchers implanted 100 nanoliters of MWCNTs in defects (0.7 mm in diameter and 2 mm deep) created in the shin-bones of mice. After four weeks they found that the cortical bone and the bone marrow cavity were completely restored. MWCNT particles were present not only in the bone marrow but also in the bone matrix. Observation of the interface between the MWCNT particles and bone matrix disclosed that MWCNTs adhered directly to the bone itself. | |
| For the third issue, new bone formation, the scientists used bone morphogenetic proteins (BMPs), a group of proteins known for their ability to induce the formation of bone and cartilage. They made a composite consisting of BMP, MWCNTs and collagen, and implanted it in the dorsal musculature of mice. Again they found that MWCNT particles were integrated entirely into new bone and bone marrow, but also that, compared to a control group using only BMP/collagen, the MWCNTs seemed to accelerate new bone formation in response to BMP. | |
| And finally, Saito's team confirmed that hydroxyapatite was formed and crystallized on the MWCNT surface in simulated body fluid remarkably quickly, and that the MWCNTs acted as the core for the initial hydroxyapatite crystallization. | |
| "The results of these experiments demonstrate that MWCNTs possess good bone compatibility, as indicated by the minimal inflammatory reaction that we saw" says Saito. "Furthermore, MWCNTs did not inhibit bone repair, and were integrated into bone. Direct contact of MWCNTs with bone tissue is particularly important for demonstrating compatibility with good integrity of healed bone. Biomaterials to be positioned in contact with bone must show bone-tissue compatibility as well as not inhibiting bone-tissue repair. Our results thus indicate the compatibility of MWCNTs with biomaterials positioned in contact with bone and their suitability for use in regions of bone healing such as fracture sites." | |
| Saito points out that, while further studies are required to elucidate the mechanism by which MWCNTs affect bone formation, the observation that bone formation is accelerated by the addition of MWCNTs is extremely important for the medical application of MWCNTs to biomaterials concerning bone. "Our finding should facilitate development of new drug delivery systems or scaffold materials for bone regeneration using MWCNTs" he says. "Furthermore, including MWCNTs in implants for the treatment of fractures, such as plates and screws, may promote bone repair and thus facilitate rapid fracture healing." | |
| He also notes that it is necessary that other structures and sizes of CNTs should be assessed as well. "Further studies are also necessary to determine the characteristics of bone containing CNTs, including remodeling properties. In addition, the metabolism of CNTs in vivo should be studied in detail. As is true generally, safety-related testing involving carcinogenesis and other toxicity is necessary prior to CNT use in humans." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
