| Posted: Feb 27, 2017 | |
Emerging redox flow battery technology for grid-scale storage |
|
| (Nanowerk Spotlight) Flow batteries are regarded as one of the most promising energy storage technologies for stationary large-scale storage because the power capability and the energy storage capability of these storage systems can be sized independently, which benefits load balancing, peak shaving, power conversion and stand-alone power system. | |
| As an emerging rechargeable battery technology, lithium redox flow batteries (Li-RFB) represent an important advance which is distinct from conventional solid-state rechargeable batteries. In RFBs, the active materials involved in the electrode reactions are soluble. | |
| Researchers have now demonstrated an all-metallocene-based non-aqueous redox flow battery with stable cycling performance and comparable energy density with current related energy storage technologies. | |
| "Different from conventional flow battery chemistries based on metal ions, metallocenes with a metal center sandwiched between two cyclopentadienyl ligands allow more freedom to tune both the physical and chemical characteristics via molecular engineering methods," Guihua Yu, a professor in Materials Science and Engineering, Mechanical Engineering, at the Texas Materials Institute, University of Texas at Austin, explains to Nanowerk. "In our recent work we propose a prototype Li-RFB by exploiting appropriate metallocenes to serve as both cathode-active and anode-active materials." | |
| "This all-metallocene-based Li-RFB shows that organometallic materials represent an important direction towards high performance non-aqueous RFBs," he points out. | |
| The results from this interdisciplinary study – integrating organic chemistry, physical chemistry and material science – have been published in Energy & Environmental Science ("A high-performance all-metallocene-based, non-aqueous redox flow battery"). | |
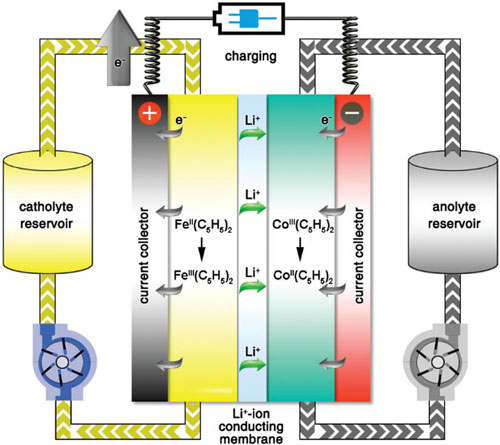 |
|
| Schematic illustration of the working principle of the proposed all-metallocene-based lithium redox flow battery during the charging process. (© The Royal Society of Chemistry) (click on image to enlarge) | |
| The most exciting result of this research is the development of the cost-effective redox flow battery solely based on organometallic redox species, which holds promise in large-scale energy storage. | |
| "To achieve wide implementation of sustainable energy systems, our research group initially investigated a series of organic- and organometallic-based green redox flow batteries, including the ferrocene-based non-aqueous redox flow battery (Angew Chem Int Ed, "Sustainable Electrical Energy Storage through the Ferrocene/Ferrocenium Redox Reaction in Aprotic Electrolyte"), and quinone-based hybrid redox flow battery (Angew Chem Int Ed, "A Bio-Inspired, Heavy-Metal-Free, Dual-Electrolyte Liquid Battery towards Sustainable Energy Storage")," recounts Yu. | |
| In light of these works, in their present research the team made a step further by developing an organic full flow battery using metallocenes as both catholyte and anolyte redox species for Li-based non-aqueous redox flow batteries. | |
| This novel energy storage system combines the concept and advantage of Li-ion batteries and redox flow batteries, and steps further beyond the conventional intercalation electrode materials and the conventional redox flow batteries. | |
| Compared with conventional redox flow batteries in water, the working potential of metallocene-based non-aqueous redox flow battery can be lifted. Considering other benefits, such as high energy density, environmental-benignity and potentially low cost, this novel redox flow battery is highly suitable for stationary energy storage. | |
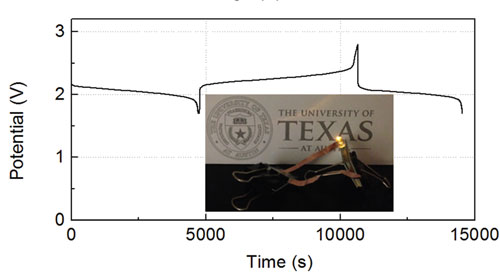 |
|
| Voltage profile of the full battery using FeCp2, FeCp2PF6 in DMF based catholyte and CoCp'2 in DOL based anolyte at 0.4C. The inset photograph shows that this modified full battery can provide a potential high enough to power a yellow LED bulb. (© The Royal Society of Chemistry) | |
| "As organometallic redox species, the cyclopentadienyl ligands in metallocene provide more flexibility to tune both the physical and chemical properties of electroactive materials," notes Yu. "In this present paper, we already tuned the redox potential by functionalizing electron-donating or electron-withdrawing groups. In the next stage, we will try to increase the solubility of metallocenes by further chemical modifications such as adding long chain ethers, which would be beneficial to realize even higher energy density." | |
| He points out, though, that the power density of the current device is limited by the LISICON-type solid electrolyte as separator, as was used in this study. Further development of advanced separators with higher conductivity and stability can help alleviate this problem. | |
| "The redox reactions of metallocenes still rely on the metals, and adoption of organic species to construct metal-free redox flow battery will be an important future research direction," concludes Yu. "This field is still in its infancy and some specific challenges include instable organic intermediates, high-cost of organic synthesis using precious metal-based catalysts, and lack of organic anode materials." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
