| Posted: Jun 15, 2017 | |
Capturing carbon dioxide and storing methane with metal-organic frameworks |
|
| (Nanowerk Spotlight) Metal-organic frameworks (MOFs) are well-ordered, lattice-like crystals. The nodes of the lattices are metals – such as copper, zinc, nickel or cobalt – which are connected by organic molecules. | |
| The most impressive features of MOFs are their extremely high surface area (up to 10000 m2 g-1), high porosity (up to 90%) and tunable pore sizes, which are remarkable advantages over other porous materials (e.g., zeolites and carbons). | |
| With their special structure and large surface area, MOFs open up new opportunities for alternative systems for gas and energy storage (e.g. carbon dioxide and hydrogen storage), in catalysis, chemical sensing, as nanoreactors, and in drug delivery, making them hugely interesting for both university research and industry. | |
| As two major low-carbon energy technologies, carbon dioxide (CO2) capture and methane (CH4) storage are facing the same challenge, i.e., the lack of capable sorbents. Many studies have suggested that MOFs are promising candidate materials. However, no MOFs have been sufficiently developed for these two applications by far. | |
| A recent review article in Advanced Energy Materials ("Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage") by scientists at the Chinese Academy of Sciences, focuses on the recent progress of development of MOFs for CO2 capture at low pressures relevant to the post-combustion conditions and CH4 storage. | |
| They first provide an overview and describe some fundamental concepts for the CO2 capture technology. Then, they highlight six promising strategies being applied to improve the CO2 capture performance of MOFs: MOFs with open metal sites; doping metals; nitrogen-rich MOFs; amine-functionalization; pore/window control; and MOFs-based composites. They discuss the individual advantages of each strategy. | |
| After this overview, they discuss the conventional and novel techniques for regenerating MOFs. They present the development of MOFs for CH4 storage mainly after the year of 2014, because a comprehensive review focusing on this topic was reported in 2014 (Chemical Society Reviews, "Methane storage in metal–organic frameworks"). | |
| In their review, the authors pay particular attention to those MOFs that can potentially meet the target of CH4 storage capacity set by U.S. Department of Energy. | |
| In the final part of the review, they then draw some conclusions and provide some outlook for the future research on both CO2 capture and CH4 storage. | |
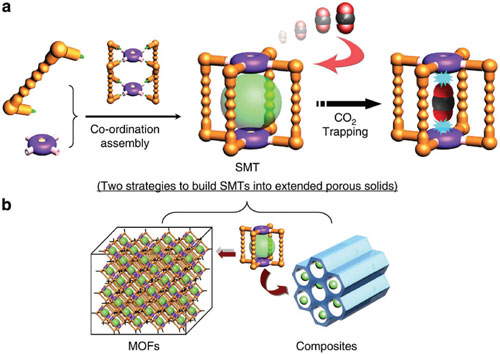 |
|
| Schematic representation of the design and construction of a single-molecule trap (SMT) for CO2 adsorption. Organic linker is shown in orange, and the metal node with an open adsorption site is shown in purple and yellow; green spheres represent the cavity for CO2 adsorption. b) Two proposed strategies for building a pre-designed SMT into extended porous solids: assembly of the SMTs by ligand extension and accommodation of SMTs into other porous materials. (© Nature Publishing Group) (click on image to enlarge) | |
| Because of the different operation conditions, the strategies to improve the performance of MOFs for CO2 capture and CH4 storage are rather different. | |
| For CO2 capture from the major emission source, post-combustion flue gas, the key to improve the capacity is to enhance the interaction of CO2 with the frameworks. In this review, the researchers have selected and reviewed several approaches that have been applied to improve the CO2 adsorption capacity of MOFs at low pressures. | |
| They suggest that a combination of multiple strategies, such as creating open metal sites, pore size control and amine-functionalization, should be delicately deployed to achieve the MOFs with desired CO2 capture ability. | |
| Although extremely high surface area and pore volume are not mandatory for CO2 capture at low pressures, sufficient binding sites that are usually determined by the surface area and pore volume, are still necessary. | |
| In addition to the adsorption performance, the regeneration cost and the influence of water and other impurity should be carefully considered for practical use. | |
| Unlike the guiding rules for developing CO2 capture materials, it is suggested to design a MOF with low CH4 uptake at low pressures but high uptake at high pressures, for CH4 storage. Apparently, large surface area and pore volume are critical parameters for CH4 storage. | |
| Concluding their review, the authors propose that more attention should be paid to the MOFs with flexible structures. "In addition, the impurity of natural gas and thermal effects should also be considered. To finally achieve the practical application of MOFs, much efforts should be taken to reduce the synthesis cost of MOFs and scale up the production of MOFs." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
