| Posted: Feb 27, 2008 | |
Mathematical engines of nanomedicine |
|
| (Nanowerk Spotlight) The process of bringing a major new drug to market, from discovery to marketing, takes about 10-12 years and costs an average of $500-$800 million in industrialized countries. And still, most drugs fail before they even make it to market. About 80 percent of drugs never make it through their clinical trials. Of the medications that actually enter consumer use, an average of just 60 percent provide therapeutic benefits to patients. | |
| For a pharmaceutical company the results of the process designing new drugs leads to a library of novel compounds that are created with a specific goal, a given set of criteria. Often these criteria include the selectivity for a particular known receptor. A new drug treatment can be discovered by testing those drugs on other receptors by trial and error. Since this is a very expensive approach, pharma companies have developed sophisticated computer models that help reduce the risk and uncertainty inherent in the drug-development process. Here, one starts with a computer model of the structure of a receptor and a drug. | |
| The goal is to predict by simulation how a drug will dock (interact with a receptor), or how the receptor will fold. Drug design based on mathematical models will also become a massive task within the emerging field of nanomedicine. Although nanotechnology offers great visions of improved, personalized treatment of disease, at the same time it renders the problem of selecting the candidates for biological testing astronomically more complex. The new notion of 'design maps' for nanovectors - similar to the concept of the periodic table for chemical elements - could provide guidance for the development of optimized injectable nanocarriers through mathematical modeling. | |
| "The number of potential combinatorial variations that can be developed by choosing different nanoparticle core materials, targeting moieties, and payload molecules is very large" Prof. Mauro Ferrari tells Nanowerk. "It is easy to compile a catalogue of 100 realistic choices in each of these entry categories. Their combination results in 1 million possible candidates. Choosing just 10 different particle diameters in the range of say 10-500 nm, the total number rises to 10 million. To give the fullest power to the method, each of the 1 million molecules in an a priori, 'conventional' combinatorial library may be used as payload: molecular docking for specificity becomes less stringent a criterion if the target selectivity is accomplished by the nanovector, and not solely the drug. The total number of candidates is now 100 billion. Even more awe-inspiring numbers are obtained by considering variations in shape, multiple targeting moieties, biological barriers, avoidance mechanisms, and multiple payloads." | |
|
Ferrari, the Director of the Research Center for NanoMedicine in The Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases at The University of Texas Health Science Center at Houston, points out that the problem – and, with it, the great opportunity for the mathematically inclined – is that there exist no consensus on nanovector selection criteria, as there are for drug libraries. In a recent essay in Small ("The Mathematical Engines of Nanomedicine") Ferrari shares his opinions of the fundamental roles that mathematics plays in integrating the various disciplines that make up nanomedicine. Ferrari's group is the first to use mathematics to guide nanotechnology development in drug delivery and nanomedical formulations, in effect bringing nanomedicine closer to the pharma world, where computer modeling has been used for a long time. "Rather than focusing on molecular docking, we started by considering three fundamental processes in the journey that takes an intravascularly injected nanovector from convective transport in the blood stream to its cellular target on and beyond the vascular endothelial wall" says Ferrari. "These processes are: margination; firm adhesion by specific and nonspecific means; and cellular uptake." |
|
| By individually analyzing these three processes as a function of the nanovector design parameters (size, shape, surface properties, bulk properties, surface density of targeting moieties) and the biological characteristics of the target lesion in the body (receptor density, blood-flow descriptors, wall permeability) the researchers achieved results and methods that can be used to analyze and design nanovectors of specific overall margination, adhesion, and cell-uptake properties. | |
| Analyzing these results, the University of Texas scientists developed the concept of design maps for nanovectors to be used as a preliminary reference for choosing the properties of the nanoparticle as a function of physiological parameters at the site of desired adhesion within the target. An example of this concept has been described in a recent paper in Biomaterials ("Design maps for nanoparticles targeting the diseased microvasculature"). | |
| Ferrari explains that this approach is somewhat similar to the concept of the periodic table of chemical elements, but more akin to phase diagrams in their graphic presentation. "The design maps provide guidance for the development of optimized injectable nanocarriers and are for the time being limited to the combination of adhesion and endocytosis. These maps and their more sophisticated evolution that will be developed over time will allow for therapeutic regimens to be personalized to individual cases, resulting in greater efficacy in the fight against disease and a reduced burden of undesired and harmful side effects. | |
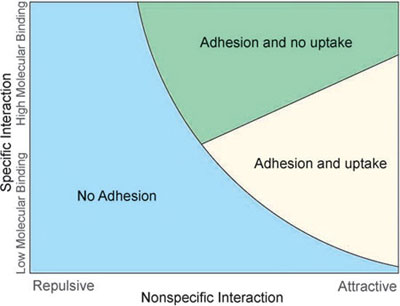 |
|
| A drug-carrying nanovector can be designed for adhesion, cellular uptake, or a combination thereof, by judiciously choosing its physical properties and geometry for a given set of biological properties of the target tissue. (Reprinted with permission from Wiley-VCH Verlag) | |
| "The design maps are but the first steps of a long journey, yet they are along a path charted by the power of mathematics to act as a compass, pointing the way to the right location, deep within a forest of extraordinary complexity, where each buried treasure corresponds to advances against human disease and suffering." he says. | |
| Led by Paolo Decuzzi, a visiting associate professor, Ferrari's group looked into ways to mathematically model nanovector selection and to identify suitable selection criteria. Not surprisingly, they found that there is no generally single best design for a nanoparticle; but they did find that there is an "absolute worst". | |
| "We discovered that nanoparticles of spherical shape with diameters of about 100 nm have the very worst margination properties, that is, they have the lowest likelihood of encountering the conjugate antigen on the target endothelium, among all spherical nanoparticles" Ferrari says. "Similarly, they have the absolute worst likelihood of penetrating through the vascular fenestrations, which make the cancer neovasculature more permeable, and thereby provide the mechanism for differentially favorable accumulation of the drugs into the target tumor. Furthermore, spherical particles perform the absolute worst in terms of adherence to the endothelial wall ? a requirement for the targeted delivery of a therapeutic payload." | |
| The bad news here is that these results indicate that almost all the nanocarriers that are in the clinic or in the preclinical pipeline are basically the worst possible size and shape for their intended purpose. The good news is that, nevertheless, these nanovectors already have proven quite successful in practice. "The pleasant nature of our unpleasant discoveries is that even small nanoparticle design improvements, fueled by mathematical analysis, will lead to greater success in the fight against human disease" says Ferrari. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
