| Posted: Oct 23, 2017 | |
Exploring the crucial role of biomolecular coronas for nanoparticle-cell interactions |
|
| (Nanowerk Spotlight) When nanoparticles enter a biological environment – e.g. human blood – they come into immediate contact with various biomolecules, such as proteins. These biomolecules form a coating layer on the nanoparticle surface – the so-called biomolecular corona – thereby imparting a unique biological identity to the nanoparticle, which could be very different from the pristine nanoparticle surface. | |
| This is the reason why biological responses to nanoparticles are strongly dependent on the type and amount of associated proteins in the composition of the biomolecular corona (read more: "Personalized protein coronas result in different therapeutic or toxic impacts of identical nanoparticles"). | |
| In new work to be reported in Nanoscale ("Apolipoprotein-enriched biomolecular corona switches the cellular uptake mechanism and trafficking pathway of lipid nanoparticles"), researchers have explored cellular uptake, endocytic pathways, and intracellular dynamics of nanoparticles in HeLa cells, both in absence and presence of biomolecular corona from human plasma. | |
| They found that the biomolecular corona could act as a personalized 'endogenous trigger' affecting off-target interactions and controlling the indication for disease of clinically approved formulations. | |
 |
|
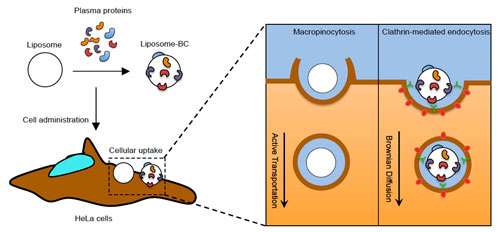
| Summary of the present understanding of the role played by biomolecular corona (BC) on the multicomponent liposome association with HeLa cells. (A) Following exposure to plasma proteins, liposome surface is decorated by a BC, which depends on several factors such as liposome's physiochemical properties (i.e. surface chemistry, size, charge etc.), protein source (e.g. human plasma vs. mouse plasma) and environmental factors (i.e. temperature, pH, etc.). For the sake of clarify, the illustration underlines that proteins cover liposome’ surface entirely, but, for simplicity of representation, it left the liposome surface only partly decorated by plasma proteins. (B) When particles are given to HeLa cells, they are efficiently internalized with cellular uptake being higher than 90% as quantified by flow cytometry. Despite similar levels of cellular uptake, BC promotes a neat switch of cell entry mechanism of liposomes, from macropinocytosis to clathrin-dependent endocytosis (C). BC has a major impact on the intracellular dynamics of liposomes. These results highlight the crucial role of BC as an intrinsic trigger of specific nanoparticle-cell interactions and biological responses. (© Royal Society of Chemistry) (click on image to enlarge) | |
| "Therapeutic failure of targeted liposomes renewed questions about understanding limitations in liposome-based drug development," Morteza Mahmoudi, Director of and Principal Investigator at the NanoBio Interactions Laboratory at Tehran University of Medical Sciences, tells Nanowerk. "In our work we have, for the first time, quantitatively demonstrated the ability of the biomolecular corona to switch the mechanism of cellular uptake of liposomes." | |
| Mahmoudi and his co-authors believe that mechanistic investigation of the biomolecular corona could contribute to a better understanding of the poor success of targeted liposomal technology. | |
| They hope that their results will contribute to the design of specific targeted nanoformulations to exploit specific cellular pathways of interest. In this way, physiological response of liposomes – bio-distribution, targeting ability etc. – could be finely tuned. This aspect may have a dramatic application in the emerging field of personalized nanomedicine. | |
| "Some of us have shown that alterations in concentration and structure of plasma proteins as those produced by clinical manifestations of disease lead to formation of 'personalized biomolecular corona'," notes Giulio Caracciolo, an Associate Professor and Principal Investigator of the Nanodelivery Lab, Department of Molecular Medicine ate Sapienza University of Rome, and senior author of the paper. "The personalized biomolecular corona may contribute to better explain why patients display different susceptibility and therapeutic responses to the same drug. This aspect deserves further investigation." | |
| Among other implications, this means that various cell types may employ different pathways to internalize nanoparticles with their own personalized biomolecular corona. | |
| "Clearly, results of our present investigation cannot fully account for the complexity of the in vivo interactions between liposomes and cells," the researchers caution. "To achieve this, will require comprehensive studies entailing libraries of liposomes, biomolecular coronas, and cells." | |
| "Results of our study open the fascinating possibility to manipulate the biomolecular corona by liposome design," they conclude. "This is not an easy task, but it could represent a turning point in the development of novel liposome-based targeting strategies for personalized nanomedicine." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
