| Posted: Jan 26, 2018 | |
Helical nanopropellers to measure local viscosity in a fluid |
|
| (Nanowerk Spotlight) In medical applications, artificial micro/nanorobots have gained a lot of attention over the last decade due to their promise to behave as ingestible vehicles of drug delivery to the deep tissue regions. This can have a significant impact in the field of cancer therapeutics for example, where the drug can be delivered to the exact location of the diseased tissue with very high efficiency. | |
| Researchers find magnetic helical nanomachines, that mimic the swimming characteristics of E. coli bacteria, to be particularly promising because of their extremely small size (0.5 to 3 µm) and their capability of navigating in various biological fluids like human blood and even in the peritoneal cavity of live mice. | |
| The field of nanomachines has developed rapidly over the last few years, with several groups exploring new methods of navigation and demonstrating their potential benefits as therapeutic tools. In the future, these nanomachines could be used in a clinical environment, where they are injected close to a specific diseased site and they are navigated to a deep location of a tissue in a completely untethered and safe manner. The nanomachines can then perform tasks like sensing or therapy at the particular site, without affecting the functionality of adjoining cells and tissues. | |
| New work, reported in Advanced Functional Materials ("Helical Nanomachines as Mobile Viscometers"), extends the possibility of using helical nanomachines as a tool to measure the localized mechanical properties of a heterogeneous environment that is ubiquitous in biological systems. | |
| This technique can be useful to gain valuable insights into the physiological changes of a cell in response to a disease or a drug, leading to better therapeutics. | |
 |
|
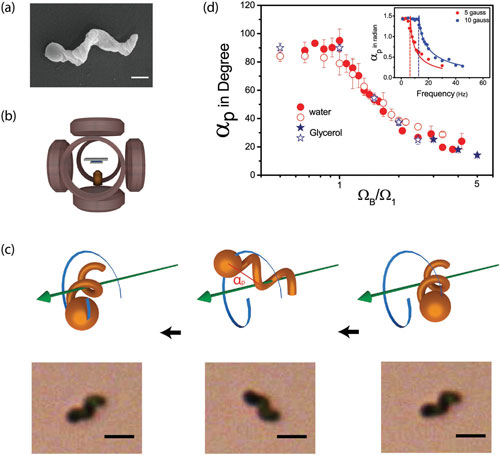
| Experimental setup and determination of precession angle. a) SEM of a nanomachine (scale bar: 500 nm). Thin film of the magnetic material was deposited after the helices were laid down on a substrate. b) Schematic of the microfluidic chamber placed inside a triaxial Helmholtz coil, and c) schematic and experimental photographs of a precessing nanomachine (scale bar: 2 µm). d) Measured angle of precession versus normalized frequency ΩB/Ω1 for water (1 cP) and glycerol (50% v/v ∼ 8 cP) at different magnetic field strengths. The inset shows αp versus ΩB at two magnetic fields; indeed, the critical frequency Ω1 ∼ 13 Hz at 10 G was found to vary linearly with the field strength. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| "Helical nanomachines that have been so far utilized as carriers of therapeutics to specific cells, can now use mechanical sensing for potential disease diagnostic purposes as well," Dr. Arijit Ghosh, the paper's first author, tells Nanowerk. | |
| In this paper, a team from the Indian Institute of Science (IIS) have shown for the first time that artificial helical nanomachines can be used to measure the local viscosity of a heterogeneous media with speed and accuracy that have never been achieved with the existing methods of viscosity measurement. | |
| The helical nanomachines can give almost real-time measurements of the local viscosity as they are navigated across fluid boundaries. The capabilities of untethered navigation using magnetic fields and the viscosity measurement empowers the nanomachines to create a map of the viscosity of any complex environment like the inside of a living cell. | |
| "This technique of creating a viscosity map can provide significant insight into microfluidic environments like mixing or co-flowing fluids, polymerization and gelation," explains Ghosh. "It can also bring out significant information of the changes in the physicochemical properties of the inside of a cell under the influence of a drug or various other physiological conditions. Lastly, the technique shows the potential of using nanomachines for in vivo detection of diseases using mechanical sensing as the measurement technique." | |
| The Optics, Nanostructures & Quantum Fluids Laboratory of Prof. Ambarish Ghosh at IIS is a pioneer in the field of helical magnetic nanomachines, which is currently being pursued by several research groups worldwide and holds a lot of promise for biomedicine. | |
| "We have found that the swimming dynamics of the nanomachines, that replicates the swimming of bacteria can be precisely controlled and manipulated using external magnetic fields showing 'run and tumble' kind of motion as found in bacterial systems," notes Ghosh. "We have performed the characterization of this dynamic behavior using both theory and experiments which brought out the effect of changing viscosity on the dynamics of the nanomachine." | |
| Thus, the team pursued their present work with the goal of demonstrating that viscosity measurement can be indeed performed with these nanomachines in an environment that has spatial heterogeneities. | |
| The scientists show that the measurements can be performed almost in real time and a spatial map of viscosity could be created. | |
| This work also extends to a different kind of fluids called shear thinning fluids that are similar to blood. The IIS researchers collaborated with Technion University, Israel to understand the dynamics of the nanomachines in shear thinning fluids, which could also be successfully characterized. In addition, the nanomachines were found to be sensitive enough to measure the small variations in viscosity due to temperature changes. | |
| As a next step, the scientists are planning to use the helical nanomachines to do viscosity measurements inside live cells under different physicochemical cues. | |
| They are also trying to extend the measurement method to the case of viscoelastic fluids or fluids with a memory, that are ubiquitously found in nature, particularly in biological systems. | |
| Potentially the technique can one day be utilized for the disease diagnosis and therapy of a localized tissue or cell in a deep in vivo environment, where it is impossible to reach in a non-invasive manner. | |
| "The main challenges for the future of autonomous therapeutic nanomachines are imaging and tracking of these micro/nano scale machines in in vivo locations, successful navigation through the treacherous paths in a tissue using external forces, without losing the payloads, and biodegradability to eliminate the potential risks if they are lost in the body," concludes Ghosh. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
