| Posted: Apr 17, 2018 | |
Graphene-oxide-based membranes for large-scale energy storage systems |
|
| (Nanowerk Spotlight) As a promising large-scale energy storage technology, redox flow batteries (RFBs) are attracting increasingly more research attention. | |
| RFBs are distinct from conventional solid-state rechargeable batteries in that the active materials involved in the electrode reactions are soluble. Though much progress has been made in identifying redox species for potential large-scale RFBs, one key limitation for their further development lies in finding low-cost and high-performance separators/membranes. | |
| "For RFB separators, the essential requirement is achieving high ionic conductivity with minimal cross-over at low cost," Guihua Yu, a professor in Materials Science and Engineering, Mechanical Engineering, at the Texas Materials Institute, University of Texas at Austin, tells Nanowerk. "Commercial membranes (Nafion) have high proton conductivity, but the production process is fairly laborious and expensive, accounting for almost 40% of the stack cost in RFBs, and the crossover problem is sometimes inevitable. Therefore, a new class of alternative membranes with facile production processes and competitive ionic conductivity are critically needed." | |
| In a new study, published in Chem ("Enabling Graphene-Oxide-Based Membranes for Large-Scale Energy Storage by Controlling Hydrophilic Microstructures"), Yu and his team demonstrate a proof-of-concept graphene oxide (GO) membrane as separator for large-scale energy RFBs. Their work shows that the two-dimensional (2D) nanochannel structure and low frictional water flow inside micrometer-thick GO laminates make this material an attractive candidate membrane for large-scale energy storage systems. | |
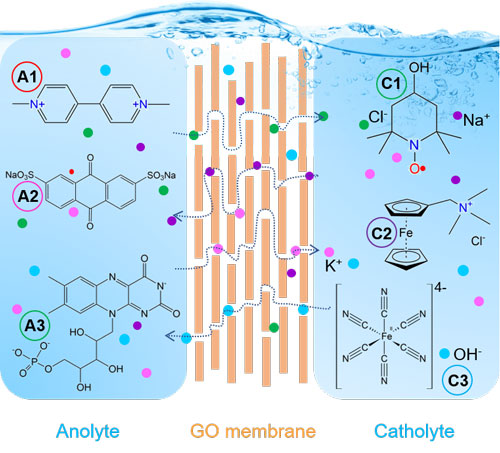 |
|
| Schematic illustration of the working mechanism of graphene oxide membranes for redox flow batteries. (© CellPress) | |
| GO laminate membranes offer tunable interlayer spacing and can act as molecular or ionic sieves to prevent the crossover of large-sized redox species. Exploring GO membranes as RFB separators, the team found that they achieve high rejection of large molecules or ions and high ionic conductivity at the same time. | |
| Moreover, changing the degree of oxidation or using bacterial cellulose as additional filling component can further adjust the microstructure, mechanical stability and ion transport behavior. | |
| "By designing large-size differences between charge carriers and redox species, GO membranes can achieve high rejection and high ionic conductivity in various redox flow battery systems," explains Yu. "Furthermore, tailoring the degree of oxidation or using bacterial cellulose as the hybrid component demonstrates the flexibility to tune the microstructure and ionic transport of GO-based membranes. Finally, RFBs with GO-based separators show a similar charge/discharge profile to commercial Nafion 212 and achieve a stable cycling performance with a high coulombic efficiency of about 98%." | |
| Yu notes that the production of GO nanosheets can be low-cost and large-scale. "Although the stability and performance of GO membranes in the flow mode still need to be further improved, our proof-of-concept demonstration of using GO membranes with tunable interlayer space, versatile chemical modification and rational composite design, provides useful guidelines for future development of next-generation functional separators for potentially large-scale energy storage systems." | |
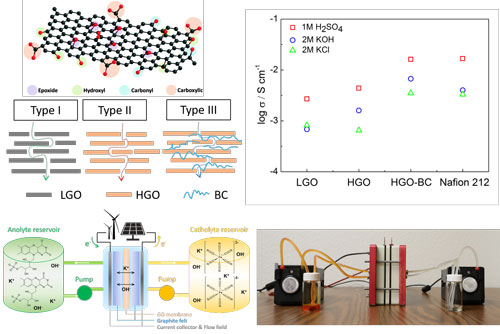 |
|
| The design of prototype redox flow battery using GO-based membranes. Three specific microstructures of GO-based membranes. (LGO: lightly oxidized graphene oxide sheet; HGO is highly oxidized graphene oxide sheet; BC is bacterial cellulose nanofiber.) Ionic conductivity in 1M H2SO4, 2M KOH and 2M KCl aqueous solutions. (© CellPress) (click on image to enlarge) | |
| The key for making GO-based membranes work for RFBs is to utilize the 2D nanofluidic structure from the stacking of GO sheets to conduct charge carriers. | |
| "This is a fundamentally new type of membrane system compared to the polymer-based membranes for vanadium ions," Yu points out. "A similar membrane structure based on GO sheets has already been explored and studied in ionic and molecular sieving and other environmental applications and shows great potential to achieve the selection of different molecules." | |
| The scientists are planning to extend the concept of 2D nanochannel structures to other materials in order to design more robust membranes since the mechanical strength of GO membranes, especially in flowing liquid, is not exceptionally high. | |
| They are also considering other new types of membranes for RFBs based on bio-derived materials with hydrophilic groups, which potentially could lead to low-cost and high-performance membranes with good mechanical properties. | |
| "A deeper understanding of ionic transport mechanism and blocking of redox species in novel types of membranes with specific nanostructures will be an interesting research direction," Yu concludes. "Apart from functionality, it is also important to examine the scalability and cost implications of new-type membranes in order to study their usefulness in practical applications." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
